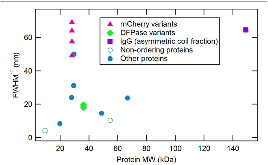
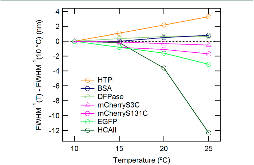
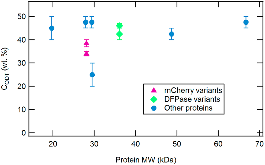
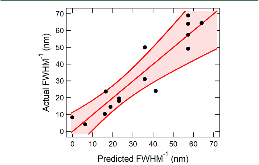
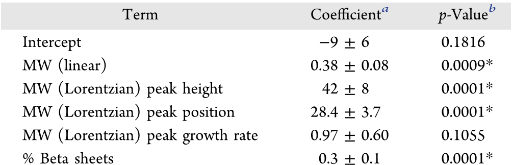
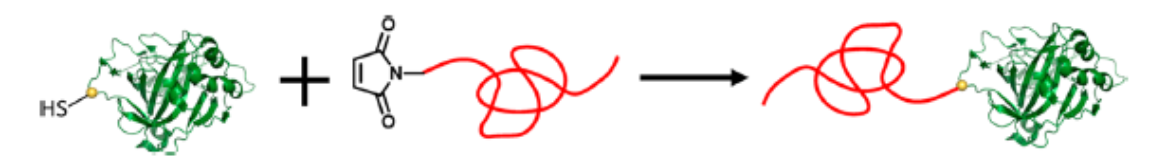|
Crystal Structures of Myoglobin-Ligand Complexes at Near-Atomic Resolution
|
journal
|
October 1999 |
|
The Nature of Protein Interactions Governing Globular Protein–Polymer Block Copolymer Self-Assembly
|
journal
|
March 2014 |
|
Refined Structure of an Intact IgG2a Monoclonal Antibody,
|
journal
|
February 1997 |
|
Tungstate as a Transition State Analog for Catalysis by Alkaline Phosphatase
|
journal
|
July 2016 |
|
Structural and immunologic characterization of bovine, horse, and rabbit serum albumins
|
journal
|
October 2012 |
|
Nanopatterned Protein Films Directed by Ionic Complexation with Water-Soluble Diblock Copolymers
|
journal
|
May 2012 |
|
Phase transitions in concentrated solution self-assembly of globular protein–polymer block copolymers
|
journal
|
January 2013 |
|
X-ray Crystallographic Studies of Alanine-65 Variants of Carbonic Anhydrase II Reveal the Structural Basis of Compromised Proton Transfer in Catalysis
|
journal
|
January 1996 |
|
Self-assembly of rod–coil block copolymers
|
journal
|
July 2008 |
|
Steric Crowding Effects on Target Detection in an Affinity Biosensor
|
journal
|
April 2007 |
|
The Effect of Protein Electrostatic Interactions on Globular Protein–Polymer Block Copolymer Self-Assembly
|
journal
|
August 2016 |
|
Progress in enzyme immobilization in ordered mesoporous materials and related applications
|
journal
|
January 2013 |
|
Microscale Enzymatic Optical Biosensors Using Mass Transport Limiting Nanofilms. 1. Fabrication and Characterization Using Glucose as a Model Analyte
|
journal
|
January 2007 |
|
Biocatalysis in non-conventional media—ionic liquids, supercritical fluids and the gas phase
|
journal
|
January 2007 |
|
Temperature-Regulated Activity of Responsive Polymer−Protein Conjugates Prepared by Grafting-from via RAFT Polymerization
|
journal
|
August 2008 |
|
Crystal Structures of Human Cytochrome P450 3A4 Bound to Metyrapone and Progesterone
|
journal
|
July 2004 |
|
Hopping Diffusion of Nanoparticles in Polymer Matrices
|
text
|
January 2015 |
|
Three-Dimensional Ordered Antibody Arrays Through Self-Assembly of Antibody-Polymer Conjugates
|
journal
|
December 2016 |
|
Binding of a Designed Substrate Analogue to Diisopropyl Fluorophosphatase: Implications for the Phosphotriesterase Mechanism
|
journal
|
October 2006 |
|
Predictive Self-Assembly of Polyhedra into Complex Structures
|
journal
|
July 2012 |
|
Polymer-Polymer Phase Behavior
|
journal
|
February 1991 |
|
Bioactive Protein Nanoarrays on Nickel Oxide Surfaces Formed by Dip-Pen Nanolithography
|
journal
|
February 2004 |
|
3V: cavity, channel and cleft volume calculator and extractor
|
journal
|
May 2010 |
|
Tutorial review. Oriented immobilization of antibodies and its applications in immunoassays and immunosensors
|
journal
|
January 1996 |
|
Creating Nanopatterns of His-Tagged Proteins on Surfaces by Nanoimprint Lithography Using Specific NiNTA-Histidine Interactions
|
journal
|
September 2007 |
|
Aggregation Behavior of Giant Amphiphiles Prepared by Cofactor Reconstitution
|
journal
|
August 2006 |
|
Solution structure and DNA-binding properties of a thermostable protein from the archaeon Sulfolobus solfataricus
|
journal
|
November 1994 |
|
Structure of the triosephosphate isomerase-phosphoglycolohydroxamate complex: an analog of the intermediate on the reaction pathway
|
journal
|
June 1991 |
|
Theory of Microphase Separation in Block Copolymers
|
journal
|
November 1980 |
|
Enzyme-based biofuel cells
|
journal
|
June 2007 |
|
Large-Scale Fabrication of Protein Nanoarrays Based on Nanosphere Lithography
|
journal
|
September 2005 |
|
Effect of Temperature on the Adsorption of Poly(N-isopropylacrylamide) at the Air−Solution Interface
|
journal
|
April 1999 |
|
Improved Ordering in Low Molecular Weight Protein–Polymer Conjugates Through Oligomerization of the Protein Block
|
journal
|
August 2018 |
|
On the Direct Electron Transfer, Sensing, and Enzyme Activity in the Glucose Oxidase/Carbon Nanotubes System
|
journal
|
December 2013 |
|
Stabilizing role of glutamic acid 222 in the structure of Enhanced Green Fluorescent Protein
|
journal
|
May 2011 |
|
Nanoscale Protein Patterning Using Self-Assembled Diblock Copolymers
|
journal
|
July 2005 |
|
Solid-State Nanostructured Materials from Self-Assembly of a Globular Protein–Polymer Diblock Copolymer
|
journal
|
June 2011 |
|
Synthesis of Uniform Protein−Polymer Conjugates
|
journal
|
November 2005 |
|
Topological Effects on Globular Protein-ELP Fusion Block Copolymer Self-Assembly
|
journal
|
December 2014 |
|
Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis.
|
journalarticle
|
January 2014 |
|
Immobilization as a Strategy for Improving Enzyme Properties-Application to Oxidoreductases
|
journal
|
June 2014 |
|
Block Copolymers—Designer Soft Materials
|
journal
|
February 1999 |
|
The potential of optofluidic biolasers
|
journal
|
January 2014 |
|
Direct electron transfer of glucose oxidase on carbon nanotubes
|
journal
|
August 2002 |
|
Evidence from Liposome Encapsulation for Transport-Limited Microbial Metabolism of Solid Alkanes
|
journal
|
February 1989 |
|
Novel Chromophores and Buried Charges Control Color in mFruits † , ‡
|
journal
|
August 2006 |
|
Stabilization of multimeric enzymes: Strategies to prevent subunit dissociation
|
journal
|
December 2009 |
|
Well-Defined Protein−Polymer Conjugates via in Situ RAFT Polymerization
|
journal
|
May 2007 |
|
Industrial enzyme applications
|
journal
|
August 2002 |
|
Enzymes as Working or Inspirational Electrocatalysts for Fuel Cells and Electrolysis
|
journal
|
July 2008 |
|
The architecture and function of the light-harvesting apparatus of purple bacteria: from single molecules to in vivo membranes
|
journal
|
August 2006 |
|
Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review
|
journal
|
November 2000 |
|
Potential Applications of Enzymes in Waste Treatment
|
journal
|
June 1997 |
|
The detection and identification of metal and organic pollutants in potable water using enzyme assays suitable for sensor development
|
journal
|
January 1995 |
|
Biomaterials for Mediation of Chemical and Biological Warfare Agents
|
journal
|
August 2003 |
|
Two-Dimensionally Self-Arranged Protein Nanoarrays on Diblock Copolymer Templates
|
journal
|
April 2007 |
|
Microsphere Solid-State Biolasers
|
journal
|
March 2017 |
|
Immobilized enzymes — learning from past successes and failures
|
journal
|
November 1993 |
|
Improvement of enzyme activity, stability and selectivity via immobilization techniques
|
journal
|
May 2007 |
|
Structural and functional stabilization of protein entities: state-of-the-art
|
journal
|
October 2015 |
|
Enzyme immobilization: an overview on techniques and support materials
|
journal
|
June 2012 |
|
Modifying enzyme activity and selectivity by immobilization
|
journal
|
January 2013 |
|
Control of protein immobilization: Coupling immobilization and site-directed mutagenesis to improve biocatalyst or biosensor performance
|
journal
|
February 2011 |
|
Bioactive Protein Nanoarrays on Nickel Oxide Surfaces Formed by Dip-Pen Nanolithography
|
journal
|
February 2004 |
|
Kinetic Effects on Self-Assembly and Function of Protein-Polymer Bioconjugates in Thin Films Prepared by Flow Coating
|
journal
|
November 2016 |
|
Thermoresponsive giant biohybrid amphiphiles
|
journal
|
January 2011 |
|
Highly Active Biocatalytic Coatings from Protein–Polymer Diblock Copolymers
|
journal
|
July 2015 |
|
Self-assembly of anisotropic tethered nanoparticle shape amphiphiles
|
journal
|
December 2005 |
|
Effect of polymer chemistry on globular protein–polymer block copolymer self-assembly
|
journal
|
January 2014 |
|
Self-assembly of protein-zwitterionic polymer bioconjugates into nanostructured materials
|
journal
|
January 2016 |
|
Self-Assembly of Differently Shaped Protein-Polymer Conjugates through Modification of the Bioconjugation Site
|
journal
|
June 2016 |
|
Inhibition of triosephosphate isomerase by phosphoenolpyruvate in the feedback-regulation of glycolysis
|
journal
|
March 2014 |
|
Water‐Restructuring Mutations Can Reverse the Thermodynamic Signature of Ligand Binding to Human Carbonic Anhydrase
|
journal
|
March 2017 |
|
Hydrogen atoms in protein structures: high-resolution X-ray diffraction structure of the DFPase
|
journal
|
January 2013 |
|
VADAR: a web server for quantitative evaluation of protein structure quality
|
journal
|
July 2003 |
|
Stable and unstable phases of a diblock copolymer melt
|
journal
|
April 1994 |
|
Hopping Diffusion of Nanoparticles in Polymer Matrices
|
journal
|
January 2015 |
|
Coil fraction-dependent phase behaviour of a model globular protein–polymer diblock copolymer
|
journal
|
January 2014 |