Cycling of oxyanion-forming trace elements in groundwaters from a freshwater deltaic marsh
- Tulane Univ., New Orleans, LA (United States). Dept. of Earth and Environmental Sciences; Los Alamos National Lab. (LANL), Los Alamos, NM (United States)
- Tulane Univ., New Orleans, LA (United States). Dept. of Earth and Environmental Sciences; Louisiana Universities Marine Consortium (LUMCON), Chauvin, LA (United States)
- Univ. of North Carolina, Chapel Hill, NC (United States). Dept. of Marine Sciences
- Louisiana Universities Marine Consortium (LUMCON), Chauvin, LA (United States)
- Tulane Univ., New Orleans, LA (United States). Dept. of Earth and Environmental Sciences
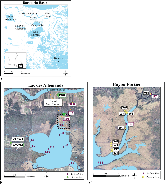
For this study, pore waters and surface waters were collected from a freshwater system in southeastern Louisiana to investigate the geochemical cycling of oxyanion-forming trace elements (i.e., Mo, W, As, V). A small bayou (Bayou Fortier) receives input from a connecting lake (Lac des Allemands) and groundwater input at the head approximately 5 km directly south of the Mississippi River. Marsh groundwaters exchange with bayou surface water but are otherwise relatively isolated from outside hydrologic forcings, such as tides, storms, and effects from local navigation canals. Rather, redox processes in the marsh groundwaters appear to drive changes in trace element concentrations. Elevated dissolved S(-II) concentrations in marsh groundwaters suggest greater reducing conditions in the late fall and winter as compared to the spring and late summer. The data suggest that reducing conditions in marsh groundwaters initiate the dissolution of Fe(III)/Mn(IV) oxide/hydroxide minerals, which releases adsorbed and/or co-precipitated trace elements into solution. Once in solution, the fate of these elements is determined by complexation with aqueous species and precipitation with iron sulfide minerals. The trace elements remain soluble in the presence of Fe(III)- and SO42-- reducing conditions, suggesting that either kinetic limitations or complexation with aqueous ligands obfuscates the correlation between V and Mo sequestration in sediments with reducing or euxinic conditions.
- Research Organization:
- Los Alamos National Lab. (LANL), Los Alamos, NM (United States)
- Sponsoring Organization:
- USDOE; National Science Foundation (NSF)
- Grant/Contract Number:
- AC52-06NA25396; EAR-1141692; EAR-1141716; EAR-1141685
- OSTI ID:
- 1463484
- Report Number(s):
- LA-UR-17-29259
- Journal Information:
- Estuarine Coastal and Shelf Science, Vol. 204, Issue C; ISSN 0272-7714
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Biogeochemical and reactive transport modeling of arsenic in groundwaters from the Mississippi River delta plain: An analog for the As-affected aquifers of South and Southeast Asia
Significance of pH and iron-sulfur chemistry for molybdenum sequestration under sulfidic conditions