Solvate Ionic Liquids at Electrified Interfaces
Abstract
Solvate ionic liquids (SILs) are a promising electrolyte for Li-ion batteries; thus, their behavior at electrified interfaces is crucial for the operation of these batteries. Here, we report molecular dynamics simulation results for a prototypical SIL of lithium triglyme bis(trifluoromethanesulfonyl)imide ([Li(G3)][TFSI]) sandwiched between electrified surfaces. At negatively charged as well as neutral electrodes, the electrolyte largely maintains the characteristics of SILs in terms of the interfacial Li+ ions’ coordination by a similar number of oxygen atoms on G3 ligands as the bulk Li+ ions. The persistence of the complex ions is attributed to the 1:1 Li–G3 ratio in bulk SILs and the fact that G3 molecules readily adapt to the interfacial environment by aligning themselves with the surface to ensure good solvation of the interfacial Li+ ions. Nevertheless, the interfacial Li+ ions also display changes of solvation from that in bulk SIL by deviating from the molecular plane formed by the oxygen atoms on G3 ligands as electrodes become more negatively charged. Using density functional theory along with natural bond orbital calculations, we examine the effects of such structural distortion on the properties of the complex cation. Both the frontier orbital energies of the complex cation and the donor–acceptor interactionsmore »
- Authors:
-
- Virginia Polytechnic Inst. and State Univ. (Virginia Tech), Blacksburg, VA (United States)
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Publication Date:
- Research Org.:
- Oak Ridge National Laboratory (ORNL), Oak Ridge, TN (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1474513
- Alternate Identifier(s):
- OSTI ID: 1482436
- Grant/Contract Number:
- AC05-00OR22725
- Resource Type:
- Accepted Manuscript
- Journal Name:
- ACS Applied Materials and Interfaces
- Additional Journal Information:
- Journal Volume: 10; Journal Issue: 38; Journal ID: ISSN 1944-8244
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 36 MATERIALS SCIENCE; 25 ENERGY STORAGE
Citation Formats
Yu, Zhou, Fang, Chao, Huang, Jingsong, Sumpter, Bobby G., and Qiao, Rui. Solvate Ionic Liquids at Electrified Interfaces. United States: N. p., 2018.
Web. doi:10.1021/acsami.8b10387.
Yu, Zhou, Fang, Chao, Huang, Jingsong, Sumpter, Bobby G., & Qiao, Rui. Solvate Ionic Liquids at Electrified Interfaces. United States. https://doi.org/10.1021/acsami.8b10387
Yu, Zhou, Fang, Chao, Huang, Jingsong, Sumpter, Bobby G., and Qiao, Rui. Wed .
"Solvate Ionic Liquids at Electrified Interfaces". United States. https://doi.org/10.1021/acsami.8b10387. https://www.osti.gov/servlets/purl/1474513.
@article{osti_1474513,
title = {Solvate Ionic Liquids at Electrified Interfaces},
author = {Yu, Zhou and Fang, Chao and Huang, Jingsong and Sumpter, Bobby G. and Qiao, Rui},
abstractNote = {Solvate ionic liquids (SILs) are a promising electrolyte for Li-ion batteries; thus, their behavior at electrified interfaces is crucial for the operation of these batteries. Here, we report molecular dynamics simulation results for a prototypical SIL of lithium triglyme bis(trifluoromethanesulfonyl)imide ([Li(G3)][TFSI]) sandwiched between electrified surfaces. At negatively charged as well as neutral electrodes, the electrolyte largely maintains the characteristics of SILs in terms of the interfacial Li+ ions’ coordination by a similar number of oxygen atoms on G3 ligands as the bulk Li+ ions. The persistence of the complex ions is attributed to the 1:1 Li–G3 ratio in bulk SILs and the fact that G3 molecules readily adapt to the interfacial environment by aligning themselves with the surface to ensure good solvation of the interfacial Li+ ions. Nevertheless, the interfacial Li+ ions also display changes of solvation from that in bulk SIL by deviating from the molecular plane formed by the oxygen atoms on G3 ligands as electrodes become more negatively charged. Using density functional theory along with natural bond orbital calculations, we examine the effects of such structural distortion on the properties of the complex cation. Both the frontier orbital energies of the complex cation and the donor–acceptor interactions between Li+ ions and G3 ligands are found to be dependent on the deviation of Li+ ions from the molecular plane of the G3 ligands, which suggests that the electrochemical reduction of Li+ ions should be facilitated by the structural distortion. These results bear important implications for the nanostructures and properties of SILs near electrified interfaces during actual operations of Li-ion batteries and serve to provide guidance toward the rational design of new SIL electrolytes.},
doi = {10.1021/acsami.8b10387},
journal = {ACS Applied Materials and Interfaces},
number = 38,
volume = 10,
place = {United States},
year = {Wed Aug 29 00:00:00 EDT 2018},
month = {Wed Aug 29 00:00:00 EDT 2018}
}
Web of Science
Figures / Tables:
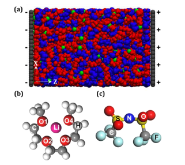 Figure 1: (a) A schematic of the simulation system composing of a slab of (b) [Li(G3)]+ complex cations and (c) [TFSI]- anions sandwiched between two charged electrodes. In (a), the blue, red, green, and gray spheres denote TFSI-ion, G3 molecule, Li+ ion, and electrode atoms, respectively. In (b-c), the magenta,more »
Figure 1: (a) A schematic of the simulation system composing of a slab of (b) [Li(G3)]+ complex cations and (c) [TFSI]- anions sandwiched between two charged electrodes. In (a), the blue, red, green, and gray spheres denote TFSI-ion, G3 molecule, Li+ ion, and electrode atoms, respectively. In (b-c), the magenta,more »
Works referenced in this record:
Ionic Liquids in Chemical Analysis
journal, July 2005
- Koel, Mihkel
- Critical Reviews in Analytical Chemistry, Vol. 35, Issue 3
Ionic Liquids at Electrified Interfaces
journal, March 2014
- Fedorov, Maxim V.; Kornyshev, Alexei A.
- Chemical Reviews, Vol. 114, Issue 5
Protic Ionic Liquids: Properties and Applications
journal, January 2008
- Greaves, Tamar L.; Drummond, Calum J.
- Chemical Reviews, Vol. 108, Issue 1, p. 206-237
Criteria for solvate ionic liquids
journal, January 2014
- Mandai, Toshihiko; Yoshida, Kazuki; Ueno, Kazuhide
- Physical Chemistry Chemical Physics, Vol. 16, Issue 19, p. 8761-8772
Change from Glyme Solutions to Quasi-ionic Liquids for Binary Mixtures Consisting of Lithium Bis(trifluoromethanesulfonyl)amide and Glymes
journal, August 2011
- Yoshida, Kazuki; Tsuchiya, Mizuho; Tachikawa, Naoki
- The Journal of Physical Chemistry C, Vol. 115, Issue 37
Oxidative-Stability Enhancement and Charge Transport Mechanism in Glyme–Lithium Salt Equimolar Complexes
journal, August 2011
- Yoshida, Kazuki; Nakamura, Megumi; Kazue, Yuichi
- Journal of the American Chemical Society, Vol. 133, Issue 33, p. 13121-13129
Anionic Effects on Solvate Ionic Liquid Electrolytes in Rechargeable Lithium–Sulfur Batteries
journal, September 2013
- Ueno, Kazuhide; Park, Jun-Woo; Yamazaki, Azusa
- The Journal of Physical Chemistry C, Vol. 117, Issue 40
Effect of Ionic Size on Solvate Stability of Glyme-Based Solvate Ionic Liquids
journal, January 2015
- Mandai, Toshihiko; Yoshida, Kazuki; Tsuzuki, Seiji
- The Journal of Physical Chemistry B, Vol. 119, Issue 4
Chelate Effects in Glyme/Lithium Bis(trifluoromethanesulfonyl)amide Solvate Ionic Liquids. I. Stability of Solvate Cations and Correlation with Electrolyte Properties
journal, May 2014
- Zhang, Ce; Ueno, Kazuhide; Yamazaki, Azusa
- The Journal of Physical Chemistry B, Vol. 118, Issue 19
Chelate Effects in Glyme/Lithium Bis(trifluoromethanesulfonyl)amide Solvate Ionic Liquids, Part 2: Importance of Solvate-Structure Stability for Electrolytes of Lithium Batteries
journal, July 2014
- Zhang, Ce; Yamazaki, Azusa; Murai, Junichi
- The Journal of Physical Chemistry C, Vol. 118, Issue 31
Solvate Ionic Liquid Electrolyte for Li–S Batteries
journal, January 2013
- Dokko, Kaoru; Tachikawa, Naoki; Yamauchi, Kento
- Journal of The Electrochemical Society, Vol. 160, Issue 8
Physicochemical Properties of Glyme–Li Salt Complexes as a New Family of Room-temperature Ionic Liquids
journal, July 2010
- Tamura, Takashi; Yoshida, Kazuki; Hachida, Takeshi
- Chemistry Letters, Vol. 39, Issue 7
Li + Local Structure in Li–Tetraglyme Solvate Ionic Liquid Revealed by Neutron Total Scattering Experiments with the 6/7 Li Isotopic Substitution Technique
journal, June 2016
- Saito, Soshi; Watanabe, Hikari; Hayashi, Yutaka
- The Journal of Physical Chemistry Letters, Vol. 7, Issue 14
Development and Testing of the OPLS All-Atom Force Field on Conformational Energetics and Properties of Organic Liquids
journal, January 1996
- Jorgensen, William L.; Maxwell, David S.; Tirado-Rives, Julian
- Journal of the American Chemical Society, Vol. 118, Issue 45
Structure and dynamics of electrical double layers in organic electrolytes
journal, January 2010
- Feng, Guang; Huang, Jingsong; Sumpter, Bobby G.
- Physical Chemistry Chemical Physics, Vol. 12, Issue 20
Computational and Experimental Study of Li-Doped Ionic Liquids at Electrified Interfaces
journal, May 2016
- Haskins, Justin B.; Wu, James J.; Lawson, John W.
- The Journal of Physical Chemistry C, Vol. 120, Issue 22
Modelling the Polymer Electrolyte/Li-Metal Interface by Molecular Dynamics simulations
journal, April 2017
- Ebadi, Mahsa; Costa, Luciano T.; Araujo, C. Moyses
- Electrochimica Acta, Vol. 234
Electrode/Electrolyte Interface in Sulfolane-Based Electrolytes for Li Ion Batteries: A Molecular Dynamics Simulation Study
journal, November 2012
- Xing, Lidan; Vatamanu, Jenel; Borodin, Oleg
- The Journal of Physical Chemistry C, Vol. 116, Issue 45
Screening of Ion–Graphene Electrode Interactions by Ionic Liquids: The Effects of Liquid Structure
journal, March 2014
- Ivaništšev, V.; Fedorov, M. V.; Lynden-Bell, R. M.
- The Journal of Physical Chemistry C, Vol. 118, Issue 11
The nanostructure of a lithium glyme solvate ionic liquid at electrified interfaces
journal, January 2017
- Coles, Samuel W.; Mishin, Maksim; Perkin, Susan
- Physical Chemistry Chemical Physics, Vol. 19, Issue 18
VMD: Visual molecular dynamics
journal, February 1996
- Humphrey, William; Dalke, Andrew; Schulten, Klaus
- Journal of Molecular Graphics, Vol. 14, Issue 1
A Second Generation Force Field for the Simulation of Proteins, Nucleic Acids, and Organic Molecules
journal, May 1995
- Cornell, Wendy D.; Cieplak, Piotr; Bayly, Christopher I.
- Journal of the American Chemical Society, Vol. 117, Issue 19
GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation
journal, February 2008
- Hess, Berk; Kutzner, Carsten; van der Spoel, David
- Journal of Chemical Theory and Computation, Vol. 4, Issue 3
Glyme–Lithium Salt Equimolar Molten Mixtures: Concentrated Solutions or Solvate Ionic Liquids?
journal, August 2012
- Ueno, Kazuhide; Yoshida, Kazuki; Tsuchiya, Mizuho
- The Journal of Physical Chemistry B, Vol. 116, Issue 36
Canonical sampling through velocity rescaling
journal, January 2007
- Bussi, Giovanni; Donadio, Davide; Parrinello, Michele
- The Journal of Chemical Physics, Vol. 126, Issue 1
Ewald summation for systems with slab geometry
journal, August 1999
- Yeh, In-Chul; Berkowitz, Max L.
- The Journal of Chemical Physics, Vol. 111, Issue 7
Density‐functional thermochemistry. III. The role of exact exchange
journal, April 1993
- Becke, Axel D.
- The Journal of Chemical Physics, Vol. 98, Issue 7, p. 5648-5652
Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18
journal, May 1980
- McLean, A. D.; Chandler, G. S.
- The Journal of Chemical Physics, Vol. 72, Issue 10, p. 5639-5648
Towards understanding the structure and capacitance of electrical double layer in ionic liquids
journal, October 2008
- Fedorov, Maxim V.; Kornyshev, Alexei A.
- Electrochimica Acta, Vol. 53, Issue 23
Microstructure and Capacitance of the Electrical Double Layers at the Interface of Ionic Liquids and Planar Electrodes
journal, February 2009
- Feng, G.; Zhang, J. S.; Qiao, R.
- The Journal of Physical Chemistry C, Vol. 113, Issue 11
Three-Dimensional Double Layers
journal, June 2014
- Kornyshev, Alexei A.; Qiao, Rui
- The Journal of Physical Chemistry C, Vol. 118, Issue 32
Modeling Insight into Battery Electrolyte Electrochemical Stability and Interfacial Structure
journal, November 2017
- Borodin, Oleg; Ren, Xiaoming; Vatamanu, Jenel
- Accounts of Chemical Research, Vol. 50, Issue 12
Structural Transitions at Ionic Liquid Interfaces
journal, December 2015
- Rotenberg, Benjamin; Salanne, Mathieu
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 24
Imidazolium Ionic Liquid Interfaces with Vapor and Graphite: Interfacial Tension and Capacitance from Coarse-Grained Molecular Simulations
journal, August 2011
- Merlet, Céline; Salanne, Mathieu; Rotenberg, Benjamin
- The Journal of Physical Chemistry C, Vol. 115, Issue 33
Molecular Simulations of the Electric Double Layer Structure, Differential Capacitance, and Charging Kinetics for N -Methyl- N -propylpyrrolidinium Bis(fluorosulfonyl)imide at Graphite Electrodes
journal, March 2011
- Vatamanu, Jenel; Borodin, Oleg; Smith, Grant D.
- The Journal of Physical Chemistry B, Vol. 115, Issue 12
Molecular dynamics simulation of the electrochemical interface between a graphite surface and the ionic liquid [BMIM][PF6]
journal, January 2009
- Kislenko, Sergey A.; Samoylov, Igor S.; Amirov, Ravil H.
- Physical Chemistry Chemical Physics, Vol. 11, Issue 27
Polarization Relaxation in an Ionic Liquid Confined between Electrified Walls
journal, February 2007
- Pinilla, Carlos; Del Pópolo, M. G.; Kohanoff, Jorge
- The Journal of Physical Chemistry B, Vol. 111, Issue 18
Double-Layer in Ionic Liquids: Paradigm Change?
journal, May 2007
- Kornyshev, Alexei A.
- The Journal of Physical Chemistry B, Vol. 111, Issue 20
Poly(a)morphic portrait of the electrical double layer in ionic liquids
journal, November 2014
- Ivaništšev, V.; O’Connor, S.; Fedorov, M. V.
- Electrochemistry Communications, Vol. 48
Calculating the Maximum Density of the Surface Packing of Ions in Ionic Liquids
journal, April 2018
- Kislenko, S. A.; Moroz, Yu. O.; Karu, K.
- Russian Journal of Physical Chemistry A, Vol. 92, Issue 5
Interfacial structure and structural forces in mixtures of ionic liquid with a polar solvent
journal, January 2018
- Coles, Samuel W.; Smith, Alexander M.; Fedorov, Maxim V.
- Faraday Discussions, Vol. 206
The Electric Double Layer Has a Life of Its Own
journal, June 2014
- Merlet, Céline; Limmer, David T.; Salanne, Mathieu
- The Journal of Physical Chemistry C, Vol. 118, Issue 32
Ion adsorption at a metallic electrode: an ab initio based simulation study
journal, September 2009
- Pounds, M.; Tazi, S.; Salanne, M.
- Journal of Physics: Condensed Matter, Vol. 21, Issue 42
Hard and Soft Acids and Bases
journal, November 1963
- Pearson, Ralph G.
- Journal of the American Chemical Society, Vol. 85, Issue 22
No rabbit ears on water. The structure of the water molecule: What should we tell the students?
journal, February 1987
- Laing, Michael
- Journal of Chemical Education, Vol. 64, Issue 2
Rabbit-ears hybrids, VSEPR sterics, and other orbital anachronisms
journal, January 2014
- Clauss, Allen D.; Nelsen, Stephen F.; Ayoub, Mohamed
- Chem. Educ. Res. Pract., Vol. 15, Issue 4
Works referencing / citing this record:
Redox-active glyme–Li tetrahalogenoferrate( iii ) solvate ionic liquids for semi-liquid lithium secondary batteries
journal, January 2020
- Kemmizaki, Yuta; Katayama, Yu; Tsutsumi, Hiromori
- RSC Advances, Vol. 10, Issue 7
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal