Increasing M2(dobdc) Loading in Selective Mixed-Matrix Membranes: A Rubber Toughening Approach
Abstract
Mixed-matrix membranes (MMMs) were formed by incorporating M2(dobdc) (M = Mg, Ni; dobdc4– = 2,5-dioxido-1,4-benzenedicarboxylate) metal–organic framework (MOF) nanoparticles in a series of poly(ether-imide) copolymers. Addition of the MOF nanoparticles improved the permeability of H2, N2, CH4, and CO2 relative to the pure copolymer by increasing gas solubility and, in most cases, diffusivity. More limited improvements in diffusivity were observed for the more strongly adsorbing gases. Because of such transport considerations, improvements in permeability and selectivity were most pronounced for H2/CH4 and H2/N2 separations. Incorporation of a greater ether content within the copolymers led to the formation of defect-free MMMs by physically sealing polymer–MOF interfacial defects, allowing higher MOF loadings to be achieved. For Mg2(dobdc), selective, defect-free films could be formed with loadings of up to 51 wt %. However, at these high loadings, films became weak and brittle. The mechanical properties of the composite materials were therefore quantified by tensile tests and compared to those of the neat polymers used commercially for membrane film formation. High contents of flexible ether units and small MOF nanoparticle sizes were found to be necessary to form strong and ductile MMMs, although clear trade-offs exist between transport performance, MOF loading, and mechanical properties.more »
- Authors:
-
- Univ. of California, Berkeley, CA (United States). Dept. of Chemistry; Massachusetts Inst. of Technology (MIT), Cambridge, MA (United States). Dept. of Chemical Engineering
- Univ. of California, Berkeley, CA (United States). Dept. of Chemical and Biomolecular Engineering
- Univ. of California, Berkeley, CA (United States). Dept. of Materials Science and Engineering; ShanghaiTech Univ., Shanghai (China). School of Physical Science and Technology
- Univ. of New South Wales, Sydney, NSW (Australia). School of Mechanical and Manufacturing Engineering
- National Energy Technology Lab. (NETL), Pittsburgh, PA (United States)
- Univ. of California, Berkeley, CA (United States). Dept. of Chemistry; Univ. of California, Berkeley, CA (United States). Dept. of Materials Science and Engineering
- Univ. of California, Berkeley, CA (United States). Dept. of Materials Science and Engineering; Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). Materials Sciences Division
- Univ. of California, Berkeley, CA (United States). Dept. of Chemistry; Univ. of California, Berkeley, CA (United States). Dept. of Chemical and Biomolecular Engineering
- Publication Date:
- Research Org.:
- National Energy Technology Laboratory (NETL), Pittsburgh, PA, Morgantown, WV (United States); Energy Frontier Research Centers (EFRC) (United States). Center for Gas Separations Relevant to Clean Energy Technologies (CGS)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1455423
- Grant/Contract Number:
- SC0001015; FE0004000
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Chemistry of Materials
- Additional Journal Information:
- Journal Volume: 30; Journal Issue: 5; Journal ID: ISSN 0897-4756
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 36 MATERIALS SCIENCE; 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Smith, Zachary P., Bachman, Jonathan E., Li, Tao, Gludovatz, Bernd, Kusuma, Victor A., Xu, Ting, Hopkinson, David P., Ritchie, Robert O., and Long, Jeffrey R. Increasing M2(dobdc) Loading in Selective Mixed-Matrix Membranes: A Rubber Toughening Approach. United States: N. p., 2018.
Web. doi:10.1021/acs.chemmater.7b02908.
Smith, Zachary P., Bachman, Jonathan E., Li, Tao, Gludovatz, Bernd, Kusuma, Victor A., Xu, Ting, Hopkinson, David P., Ritchie, Robert O., & Long, Jeffrey R. Increasing M2(dobdc) Loading in Selective Mixed-Matrix Membranes: A Rubber Toughening Approach. United States. https://doi.org/10.1021/acs.chemmater.7b02908
Smith, Zachary P., Bachman, Jonathan E., Li, Tao, Gludovatz, Bernd, Kusuma, Victor A., Xu, Ting, Hopkinson, David P., Ritchie, Robert O., and Long, Jeffrey R. Tue .
"Increasing M2(dobdc) Loading in Selective Mixed-Matrix Membranes: A Rubber Toughening Approach". United States. https://doi.org/10.1021/acs.chemmater.7b02908. https://www.osti.gov/servlets/purl/1455423.
@article{osti_1455423,
title = {Increasing M2(dobdc) Loading in Selective Mixed-Matrix Membranes: A Rubber Toughening Approach},
author = {Smith, Zachary P. and Bachman, Jonathan E. and Li, Tao and Gludovatz, Bernd and Kusuma, Victor A. and Xu, Ting and Hopkinson, David P. and Ritchie, Robert O. and Long, Jeffrey R.},
abstractNote = {Mixed-matrix membranes (MMMs) were formed by incorporating M2(dobdc) (M = Mg, Ni; dobdc4– = 2,5-dioxido-1,4-benzenedicarboxylate) metal–organic framework (MOF) nanoparticles in a series of poly(ether-imide) copolymers. Addition of the MOF nanoparticles improved the permeability of H2, N2, CH4, and CO2 relative to the pure copolymer by increasing gas solubility and, in most cases, diffusivity. More limited improvements in diffusivity were observed for the more strongly adsorbing gases. Because of such transport considerations, improvements in permeability and selectivity were most pronounced for H2/CH4 and H2/N2 separations. Incorporation of a greater ether content within the copolymers led to the formation of defect-free MMMs by physically sealing polymer–MOF interfacial defects, allowing higher MOF loadings to be achieved. For Mg2(dobdc), selective, defect-free films could be formed with loadings of up to 51 wt %. However, at these high loadings, films became weak and brittle. The mechanical properties of the composite materials were therefore quantified by tensile tests and compared to those of the neat polymers used commercially for membrane film formation. High contents of flexible ether units and small MOF nanoparticle sizes were found to be necessary to form strong and ductile MMMs, although clear trade-offs exist between transport performance, MOF loading, and mechanical properties. In conclusion, these trade-offs are critically examined to evaluate the current limitations and potential benefits to forming M2(dobdc) MMMs using this rubber toughening approach.},
doi = {10.1021/acs.chemmater.7b02908},
journal = {Chemistry of Materials},
number = 5,
volume = 30,
place = {United States},
year = {Tue Jan 30 00:00:00 EST 2018},
month = {Tue Jan 30 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
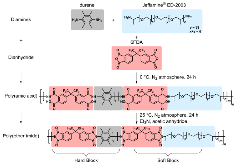 Figure 1: Synthesis of poly(ether-imide) copolymers.
Figure 1: Synthesis of poly(ether-imide) copolymers.
Works referenced in this record:
Gas Separation Membrane Materials: A Perspective
journal, September 2014
- Baker, Richard W.; Low, Bee Ting
- Macromolecules, Vol. 47, Issue 20
Natural Gas Processing with Membranes: An Overview
journal, April 2008
- Baker, Richard W.; Lokhandwala, Kaaeid
- Industrial & Engineering Chemistry Research, Vol. 47, Issue 7
The solution-diffusion model: a review
journal, November 1995
- Wijmans, J. G.; Baker, R. W.
- Journal of Membrane Science, Vol. 107, Issue 1-2
The upper bound revisited
journal, July 2008
- Robeson, Lloyd M.
- Journal of Membrane Science, Vol. 320, Issue 1-2, p. 390-400
Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation
journal, April 2007
- Chung, Tai-Shung; Jiang, Lan Ying; Li, Yi
- Progress in Polymer Science, Vol. 32, Issue 4, p. 483-507
Metal organic framework based mixed matrix membranes: An increasingly important field of research with a large application potential
journal, January 2013
- Zornoza, Beatriz; Tellez, Carlos; Coronas, Joaquin
- Microporous and Mesoporous Materials, Vol. 166
Mixed matrix membranes using carbon molecular sieves
journal, January 2003
- Vu, De Q.; Koros, William J.; Miller, Stephen J.
- Journal of Membrane Science, Vol. 211, Issue 2
Mixed matrix membranes using carbon molecular sieves
journal, January 2003
- Vu, De Q.; Koros, William J.; Miller, Stephen J.
- Journal of Membrane Science, Vol. 211, Issue 2
Membrane Gas Separation: A Review/State of the Art
journal, May 2009
- Bernardo, P.; Drioli, E.; Golemme, G.
- Industrial & Engineering Chemistry Research, Vol. 48, Issue 10
High performance ZIF-8/6FDA-DAM mixed matrix membrane for propylene/propane separations
journal, February 2012
- Zhang, Chen; Dai, Ying; Johnson, Justin R.
- Journal of Membrane Science, Vol. 389
Unexpected Molecular Sieving Properties of Zeolitic Imidazolate Framework-8
journal, July 2012
- Zhang, Chen; Lively, Ryan P.; Zhang, Ke
- The Journal of Physical Chemistry Letters, Vol. 3, Issue 16
In Situ Synthesis of Thin Zeolitic–Imidazolate Framework ZIF-8 Membranes Exhibiting Exceptionally High Propylene/Propane Separation
journal, July 2013
- Kwon, Hyuk Taek; Jeong, Hae-Kwon
- Journal of the American Chemical Society, Vol. 135, Issue 29
Sharp separation of C2/C3 hydrocarbon mixtures by zeolitic imidazolate framework-8 (ZIF-8) membranes synthesized in aqueous solutions
journal, January 2011
- Pan, Yichang; Lai, Zhiping
- Chemical Communications, Vol. 47, Issue 37
Comprehensive study of carbon dioxide adsorption in the metal–organic frameworks M 2 (dobdc) (M = Mg, Mn, Fe, Co, Ni, Cu, Zn)
journal, January 2014
- Queen, Wendy L.; Hudson, Matthew R.; Bloch, Eric D.
- Chem. Sci., Vol. 5, Issue 12
Selective adsorption of ethylene over ethane and propylene over propane in the metal–organic frameworks M2(dobdc) (M = Mg, Mn, Fe, Co, Ni, Zn)
journal, January 2013
- Geier, Stephen J.; Mason, Jarad A.; Bloch, Eric D.
- Chemical Science, Vol. 4, Issue 5
Dramatic Tuning of Carbon Dioxide Uptake via Metal Substitution in a Coordination Polymer with Cylindrical Pores
journal, August 2008
- Caskey, Stephen R.; Wong-Foy, Antek G.; Matzger, Adam J.
- Journal of the American Chemical Society, Vol. 130, Issue 33, p. 10870-10871
CO2/N2 separations with mixed-matrix membranes containing Mg2(dobdc) nanocrystals
journal, January 2013
- Bae, Tae-Hyun; Long, Jeffrey R.
- Energy & Environmental Science, Vol. 6, Issue 12
Enhanced ethylene separation and plasticization resistance in polymer membranes incorporating metal–organic framework nanocrystals
journal, April 2016
- Bachman, Jonathan E.; Smith, Zachary P.; Li, Tao
- Nature Materials, Vol. 15, Issue 8
Plasticization-resistant Ni 2 (dobdc)/polyimide composite membranes for the removal of CO 2 from natural gas
journal, January 2016
- Bachman, Jonathan E.; Long, Jeffrey R.
- Energy & Environmental Science, Vol. 9, Issue 6
Molecular sieving realized with ZIF-8/Matrimid® mixed-matrix membranes
journal, September 2010
- Ordoñez, Ma. Josephine C.; Balkus, Kenneth J.; Ferraris, John P.
- Journal of Membrane Science, Vol. 361, Issue 1-2, p. 28-37
Dynamic molecular interactions between polyurethane and ZIF-8 in a polymer-MOF nanocomposite: Microstructural, thermo-mechanical and viscoelastic effects
journal, August 2016
- Mahdi, E. M.; Tan, Jin-Chong
- Polymer, Vol. 97
Mixed-matrix membranes of zeolitic imidazolate framework (ZIF-8)/Matrimid nanocomposite: Thermo-mechanical stability and viscoelasticity underpinning membrane separation performance
journal, January 2016
- Mahdi, E. M.; Tan, Jin-Chong
- Journal of Membrane Science, Vol. 498
Physical aging in glassy mixed matrix membranes; tuning particle interaction for mechanically robust nanocomposite films
journal, January 2016
- Smith, Stefan J. D.; Lau, Cher Hon; Mardel, James I.
- Journal of Materials Chemistry A, Vol. 4, Issue 27
Effect of methyl substituents on permeability and permselectivity of gases in polyimides prepared from methyl-substituted phenylenediamines
journal, July 1992
- Tanaka, Kazuhiro; Okano, Masaaki; Toshino, Hiroyuki
- Journal of Polymer Science Part B: Polymer Physics, Vol. 30, Issue 8
Phase structure and adhesion in polymer blends: A criterion for rubber toughening
journal, November 1985
- Wu, Souheng
- Polymer, Vol. 26, Issue 12
Influence of Diffusivity and Sorption on Helium and Hydrogen Separations in Hydrocarbon, Silicon, and Fluorocarbon-Based Polymers
journal, April 2014
- Smith, Zachary P.; Tiwari, Rajkiran R.; Dose, Michelle E.
- Macromolecules, Vol. 47, Issue 9
The Time Lag in Diffusion
journal, January 1957
- Frisch, H. L.
- The Journal of Physical Chemistry, Vol. 61, Issue 1
Relationship between gas transport properties and fractional free volume determined from dielectric constant in polyimide films containing the hexafluoroisopropylidene group
journal, January 2007
- Miyata, Sou; Sato, Shuichi; Nagai, Kazukiyo
- Journal of Applied Polymer Science, Vol. 107, Issue 6
Preparation and gas separation properties of partially pyrolyzed membranes (PPMs) derived from copolyimides containing polyethylene oxide side chains
journal, August 2012
- Huertas, R. M.; Doherty, C. M.; Hill, A. J.
- Journal of Membrane Science, Vol. 409-410
Performance studies of mixed matrix membranes for gas separation: A review
journal, November 2010
- Aroon, M. A.; Ismail, A. F.; Matsuura, T.
- Separation and Purification Technology, Vol. 75, Issue 3
Poly(imide siloxane) and carbon nanotube mixed matrix membranes for gas separation
journal, May 2006
- Kim, Sangil; Pechar, Todd W.; Marand, Eva
- Desalination, Vol. 192, Issue 1-3
Gas solubility, diffusivity and permeability in poly(ethylene oxide)
journal, August 2004
- Lin, H.; Freeman, B. D.
- Journal of Membrane Science, Vol. 239, Issue 1
Thermal transitions in α,ω-diamino terminated poly(oxypropylene)-block-poly(oxyethylene)-block-poly(oxypropylene) aqueous solutions and their epoxy networks
journal, May 2005
- Gómez Ribelles, Jose Luis; Salmerón Sanchez, Manuel; de la Osa, Luis Torres
- Journal of Non-Crystalline Solids, Vol. 351, Issue 14-15
Fluorinated polycarbonates for gas separation applications
journal, September 1989
- Hellums, M. W.; Koros, W. J.; Husk, G. R.
- Journal of Membrane Science, Vol. 46, Issue 1
Predictive Models for Mixed-Matrix Membrane Performance: A Review
journal, March 2013
- Vinh-Thang, Hoang; Kaliaguine, Serge
- Chemical Reviews, Vol. 113, Issue 7
637. The stability of transition-metal complexes
journal, January 1953
- Irving, H.; Williams, R. J. P.
- Journal of the Chemical Society (Resumed), Vol. 0, Issue 0, p. 3192-3210
Hybrid membrane materials comprising organic polymers with rigid dispersed phases
journal, January 2004
- Moore, Theodore T.; Mahajan, Rajiv; Vu, De Q.
- AIChE Journal, Vol. 50, Issue 2
Diffusion of Gases in Polyethylene Terephthalate
journal, January 1963
- Michaels, Alan S.; Vieth, Wolf R.; Barrie, James A.
- Journal of Applied Physics, Vol. 34, Issue 1
Characterization of permeability and sorption in Matrimid/C60 mixed matrix membranes
journal, January 2003
- Chung, Tai-Shung; Chan, Sun Sun; Wang, Rong
- Journal of Membrane Science, Vol. 211, Issue 1
The effects of polymer chain rigidification, zeolite pore size and pore blockage on polyethersulfone (PES)-zeolite A mixed matrix membranes
journal, September 2005
- Li, Y.; Chung, T.; Cao, C.
- Journal of Membrane Science, Vol. 260, Issue 1-2
Basis of Permeability/Selectivity Tradeoff Relations in Polymeric Gas Separation Membranes
journal, January 1999
- Freeman, Benny D.
- Macromolecules, Vol. 32, Issue 2
Water Reaction Mechanism in Metal Organic Frameworks with Coordinatively Unsaturated Metal Ions: MOF-74
journal, November 2014
- Tan, Kui; Zuluaga, Sebastian; Gong, Qihan
- Chemistry of Materials, Vol. 26, Issue 23
Competitive Coadsorption of CO 2 with H 2 O, NH 3 , SO 2 , NO, NO 2 , N 2 , O 2 , and CH 4 in M-MOF-74 (M = Mg, Co, Ni): The Role of Hydrogen Bonding
journal, March 2015
- Tan, Kui; Zuluaga, Sebastian; Gong, Qihan
- Chemistry of Materials, Vol. 27, Issue 6
The diffusion time lag in polymer membranes containing adsorptive fillers
journal, January 1973
- Paul, D. R.; Kemp, D. R.
- Journal of Polymer Science: Polymer Symposia, Vol. 41, Issue 1
Enhanced H2 Adsorption in Isostructural Metal−Organic Frameworks with Open Metal Sites: Strong Dependence of the Binding Strength on Metal Ions
journal, November 2008
- Zhou, Wei; Wu, Hui; Yildirim, Taner
- Journal of the American Chemical Society, Vol. 130, Issue 46, p. 15268-15269
Computational and Experimental Studies on the Adsorption of CO, N 2 , and CO 2 on Mg-MOF-74
journal, June 2010
- Valenzano, L.; Civalleri, B.; Chavan, S.
- The Journal of Physical Chemistry C, Vol. 114, Issue 25
Evaluating metal–organic frameworks for natural gas storage
journal, January 2014
- Mason, Jarad A.; Veenstra, Mike; Long, Jeffrey R.
- Chemical Science, Vol. 5, Issue 1, p. 32-51
Evaluating metal–organic frameworks for post-combustion carbon dioxide capture via temperature swing adsorption
journal, January 2011
- Mason, Jarad A.; Sumida, Kenji; Herm, Zoey R.
- Energy & Environmental Science, Vol. 4, Issue 8, p. 3030-3040
CO2/CH4, CH4/H2 and CO2/CH4/H2 separations at high pressures using Mg2(dobdc)
journal, March 2012
- Herm, Zoey R.; Krishna, Rajamani; Long, Jeffrey R.
- Microporous and Mesoporous Materials, Vol. 151
Works referencing / citing this record:
High gas permselectivity in ZIF‐302/polyimide self‐consistent mixed‐matrix membrane
journal, September 2019
- Ghanem, Akram S.; Ba‐Shammakh, Mohamed; Usman, Muhammad
- Journal of Applied Polymer Science, Vol. 137, Issue 13
Performance of Mixed Matrix Membranes Containing Porous Two-Dimensional (2D) and Three-Dimensional (3D) Fillers for CO2 Separation: A Review
journal, July 2018
- Ahmadi, Mahdi; Janakiram, Saravanan; Dai, Zhongde
- Membranes, Vol. 8, Issue 3
2D molecular crystal lattices: advances in their synthesis, characterization, and application
journal, January 2019
- Solomos, Marina A.; Claire, F. James; Kempa, Thomas J.
- Journal of Materials Chemistry A, Vol. 7, Issue 41
Engineering of the Filler/Polymer Interface in Metal–Organic Framework‐Based Mixed‐Matrix Membranes to Enhance Gas Separation
journal, July 2019
- Ma, Liang; Svec, Frantisek; Lv, Yongqin
- Chemistry – An Asian Journal, Vol. 14, Issue 20

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal