Nucleation and phase transformation pathways in electrolyte solutions investigated by in situ microscopy techniques
Abstract
Identification of crystal nucleation and growth pathways is of fundamental importance for synthesis of functional materials, which requires control over size, orientation, polymorph, and hierarchical structure, often in the presence of additives used to tune the energy landscape defining these pathways. Here in this paper we summarize the recent progress in application of in situ TEM and AFM techniques to monitor or even tune the pathway of crystal nucleation and growth.
- Authors:
-
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States)
- Lawrence Livermore National Lab. (LLNL), Livermore, CA (United States)
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States); Univ. of Washington, Seattle, WA (United States)
- Publication Date:
- Research Org.:
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States); Lawrence Livermore National Lab. (LLNL), Livermore, CA (United States)
- Sponsoring Org.:
- USDOE National Nuclear Security Administration (NNSA); USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1437026
- Alternate Identifier(s):
- OSTI ID: 1513117; OSTI ID: 1545386
- Report Number(s):
- PNNL-SA-135089; LLNL-JRNL-741424
Journal ID: ISSN 1359-0294; PII: S1359029417301334
- Grant/Contract Number:
- AC05–76RL01830; AC52-07NA27344
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Current Opinion in Colloid & Interface Science
- Additional Journal Information:
- Journal Volume: 34; Journal Issue: C; Journal ID: ISSN 1359-0294
- Publisher:
- Elsevier
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 36 MATERIALS SCIENCE; Nucleation; Phase transformation; In situ TEM; In situ AFM; Materials science
Citation Formats
Tao, Jinhui, Nielsen, Michael H., and De Yoreo, James J. Nucleation and phase transformation pathways in electrolyte solutions investigated by in situ microscopy techniques. United States: N. p., 2018.
Web. doi:10.1016/j.cocis.2018.04.002.
Tao, Jinhui, Nielsen, Michael H., & De Yoreo, James J. Nucleation and phase transformation pathways in electrolyte solutions investigated by in situ microscopy techniques. United States. https://doi.org/10.1016/j.cocis.2018.04.002
Tao, Jinhui, Nielsen, Michael H., and De Yoreo, James J. Fri .
"Nucleation and phase transformation pathways in electrolyte solutions investigated by in situ microscopy techniques". United States. https://doi.org/10.1016/j.cocis.2018.04.002. https://www.osti.gov/servlets/purl/1437026.
@article{osti_1437026,
title = {Nucleation and phase transformation pathways in electrolyte solutions investigated by in situ microscopy techniques},
author = {Tao, Jinhui and Nielsen, Michael H. and De Yoreo, James J.},
abstractNote = {Identification of crystal nucleation and growth pathways is of fundamental importance for synthesis of functional materials, which requires control over size, orientation, polymorph, and hierarchical structure, often in the presence of additives used to tune the energy landscape defining these pathways. Here in this paper we summarize the recent progress in application of in situ TEM and AFM techniques to monitor or even tune the pathway of crystal nucleation and growth.},
doi = {10.1016/j.cocis.2018.04.002},
journal = {Current Opinion in Colloid & Interface Science},
number = C,
volume = 34,
place = {United States},
year = {Fri Apr 27 00:00:00 EDT 2018},
month = {Fri Apr 27 00:00:00 EDT 2018}
}
Free Publicly Available Full Text
Publisher's Version of Record
Other availability
Cited by: 12 works
Citation information provided by
Web of Science
Web of Science
Figures / Tables:
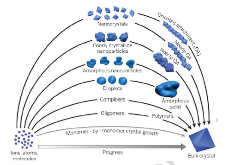 Fig. 1: Many wide-ranging crystallization pathways have been proposed and/or observed for electrolyte solutions. In contrast to monomer-by-monomer addition as envisioned in classical models of crystal growth (gray curve), crystallization can occur by the addition of higher-order species ranging from multi-ion complexes to fully formed nanocrystals. Furthermore, the final phasemore »
Fig. 1: Many wide-ranging crystallization pathways have been proposed and/or observed for electrolyte solutions. In contrast to monomer-by-monomer addition as envisioned in classical models of crystal growth (gray curve), crystallization can occur by the addition of higher-order species ranging from multi-ion complexes to fully formed nanocrystals. Furthermore, the final phasemore »
All figures and tables
(6 total)
Save to My Library
You must Sign In or Create an Account in order to save documents to your library.
Figures / Tables found in this record:
Figures/Tables have been extracted from DOE-funded journal article accepted manuscripts.

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal