23Na nuclear magnetic resonance study of yNa2S +(1-y)[xSiS2 + (1-x)PS5/2] glassy solid electrolytes
- Minnesota State Univ., Moorhead, MN (United States); Ames Lab. and Iowa State Univ., Ames, IA (United States)
- Iowa State Univ., Ames, IA (United States); Central Research and Development, St. Paul, MN (United States)
- Ames Lab. and Iowa State Univ., Ames, IA (United States)
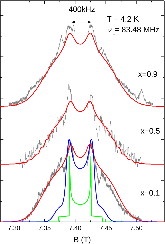
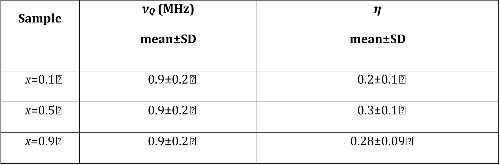
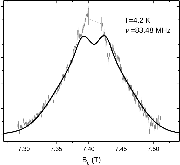
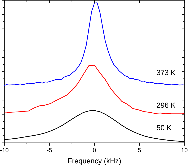
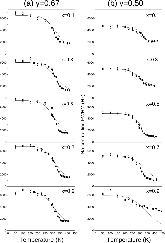
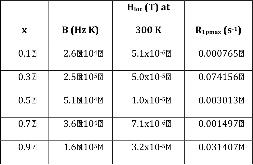
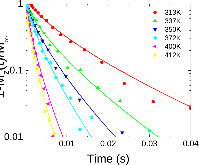
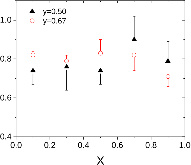
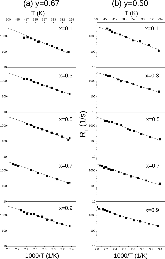
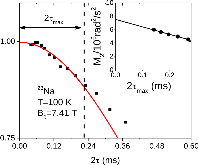
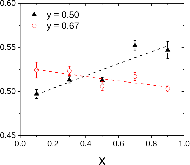
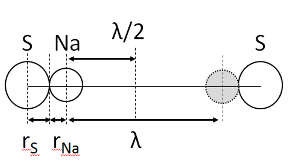
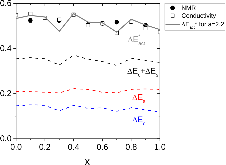
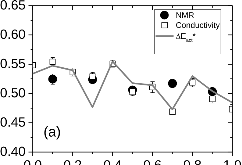
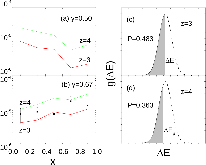
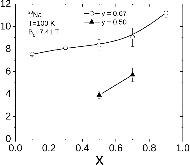
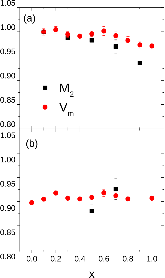
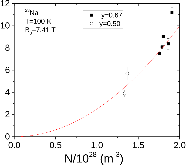
23Na NMR spin lattice relaxation times, T1, and central linewidths were obtained for yNa2S +(1-y)[xSiS2 + (1-x)PS5/2] glassy solid electrolytes for two series of glasses, y=0.5 and 0.67, and x=0.1, 0.3, 0.5, 0.7, and 0.9. No pronounced mixed glass former effect in the activation energy was recorded within experimental uncertainty for either series of glasses. Energy barriers to sodium motion were calculated using the Anderson-Stuart model for the y=0.67 sample, and the results suggested that the energy barriers as a function of composition are strongly influenced by the dielectric constant of these glasses. DC Na+ ion conductivity values calculated using NMR-derived correlation times, an available Na+ ion site coordination number in the range z=3–4, and an energy cutoff determined from the critical percolation threshold, were in agreement with the increasing trend in the experimental values for the y=0.67 glasses. Using the same model, the conductivity values were calculated for the y=0.50 glasses, which have as yet to be measured, and these revealed a decreasing conductivity as x increased. Sodium NMR second moment studies showed that the cation spatial arrangement followed a homogeneous distribution for y=0.50 and 0.67 samples over most of the composition range, but deviated significantly away from this above a sodium concentration of 1.85 ×1028 m-3, implying a tendency for sodium to cluster under these conditions.
- Research Organization:
- Ames Laboratory (AMES), Ames, IA (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES) (SC-22)
- Grant/Contract Number:
- AC02-07CH11358
- OSTI ID:
- 1542873
- Alternate ID(s):
- OSTI ID: 1532441
- Report Number(s):
- IS-J--9973
- Journal Information:
- Solid State Ionics, Journal Name: Solid State Ionics Journal Issue: C Vol. 340; ISSN 0167-2738
- Publisher:
- ElsevierCopyright Statement
- Country of Publication:
- United States
- Language:
- English