Uranium exchange kinetics in a molten LiCl-KCl/Cd system at 500 °C
- Idaho National Lab. (INL), Idaho Falls, ID (United States)
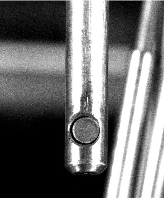
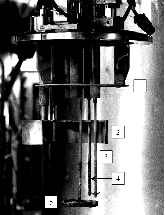
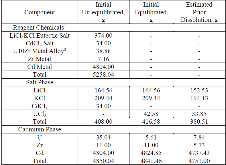
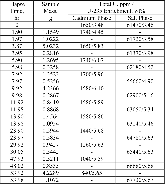
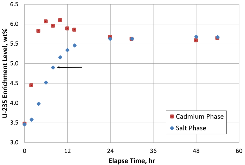
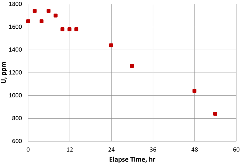
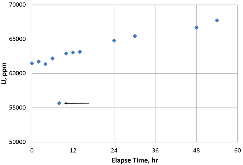
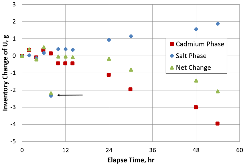
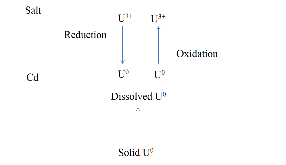
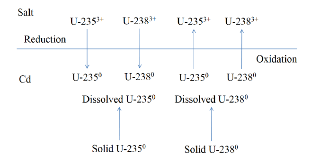
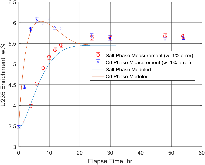
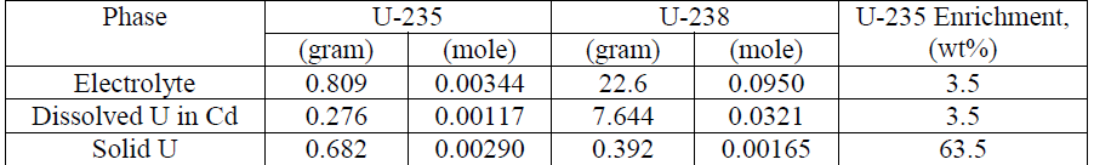
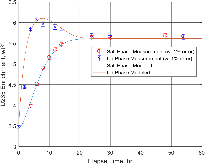
A dissolution experiment is performed in order to examine the kinetics of uranium isotope equilibration in a molten salt and cadmium system. The salt contains a solute concentration of UCl3 in LiCl-KCl eutectic and the cadmium contained dissolved uranium at 500 °C. A small piece of high enriched uranium is introduced to the cadmium, which perturbs the equilibrium with respect to the uranium enrichment of the salt and cadmium phases. The series of salt and cadmium samples trace how the enrichment of the two phases reach equilibrium (in the absence of a chemical driving force) over the course of approximately 24 h. Here, a model linking dissolution and isotope exchange kinetics is developed to track the evolution of uranium isotope composition. With the assumption of unaccounted salt mass incurred from the dated sample reporting practice, the model explains the trend of the measured uranium isotope composition well.
- Research Organization:
- Idaho National Laboratory (INL), Idaho Falls, ID (United States)
- Sponsoring Organization:
- USDOE Office of Nuclear Energy (NE)
- Grant/Contract Number:
- AC07-05ID14517
- OSTI ID:
- 1478485
- Report Number(s):
- INL/JOU--18-44721-Rev000
- Journal Information:
- Journal of Nuclear Materials, Journal Name: Journal of Nuclear Materials Journal Issue: C Vol. 508; ISSN 0022-3115
- Publisher:
- ElsevierCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Review—Electrochemical Measurements in Molten Salt Systems: A Guide and Perspective
|
journal | January 2019 |
Similar Records
Electrolysis of uranium nitride containing fission product elements (Mo, Pd, Nd) in a molten LiCl-KCl eutectic
Thermodynamic Assessment of LiCl-KCl-PuCl{sub 3} Ternary System