Oxygen Reduction on Gold Nanocrystal Surfaces in Alkaline Electrolyte: Evidence for Surface Proton Transfer Effects
- Brookhaven National Lab. (BNL), Upton, NY (United States)
- Brookhaven National Lab. (BNL), Upton, NY (United States); Columbia Univ., New York, NY (United States)
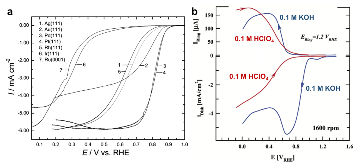
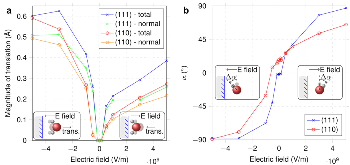
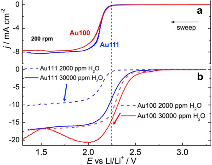
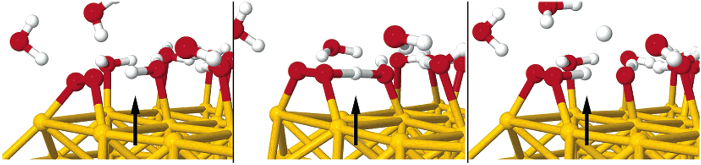
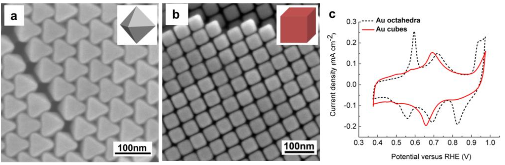
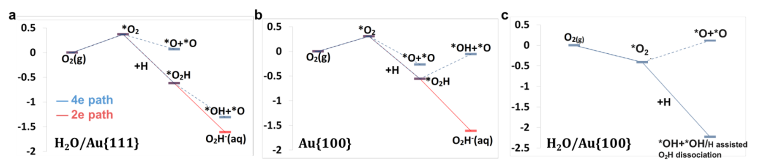
Four-electron oxygen reduction reaction (4e-ORR) pathway, as a key high-performance reaction pathway in energy conversion, has been sought after in numerous investigations on metal surfaces over the last decades. Although the surfaces of the most noble metals, including platinum and palladium, demonstrate the fullpotential- range 4e-ORR, this is not the case, for gold (Au) surfaces. The 4e-ORR is only operative on Au surfaces with {100} subfacets, e.g. Au(100), in alkaline solution, however restricted to a certain potential region at low overpotentials, while reverting to a 2e-ORR at high overpotentials. This ORR on Au(100) has been a long-standing puzzle of electrocatalysis. Hereby we review the ORR studies on Au, along with the studies of water effects on Au catalysts, and present our electrochemical results with monofacet Au nanocrystals. Finally, combining with theoretical calculations we demonstrate that surface proton transfer from co-adsorbed water plays the key role in determining the ORR mechanism on Au surfaces in base.
- Research Organization:
- Brookhaven National Laboratory (BNL), Upton, NY (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES) (SC-22)
- Grant/Contract Number:
- SC0012704
- OSTI ID:
- 1430856
- Report Number(s):
- BNL--203376-2018-JAAM
- Journal Information:
- ECS Transactions (Online), Journal Name: ECS Transactions (Online) Journal Issue: 12 Vol. 85; ISSN 1938-6737
- Publisher:
- Electrochemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Similar Records
Oxygen and hydrogen peroxide reduction on the Au(100) surface in alkaline electrolyte. The roles of surface structure and hydroxide adsorption
Mechanism of oxygen reduction reaction on Pt(111) in alkaline solution: Importance of chemisorbed water on surface