Rare-earth titanate melt structure and glass formation
Abstract
The structure of rare–earth titanate melts and glasses of composition 17RE2O3.83TiO2 have been investigated in situ by aerodynamic levitation with laser heating. Ti K–edge X–ray absorption near–edge structure (XANES) spectroscopy reveals an effect of RE cation size on mean Ti–O coordination numbers (nTiO), which increase from ~4.8(2) in glass–forming La titanate to ~5.1(2) in non–glass–forming Sc titanate liquids. We suggest that the associated increase in OTi3 triclusters in melts bearing smaller RE cations tends to inhibit glass formation. Both XANES and high–energy X–ray diffraction indicate increases in nTiO as the liquids supercool and vitrify. Results are discussed in the context of alkali and alkaline–earth titanate glasses, extending the observed dependence of nTiO on structural basicity (modifier content divided by potential) to trivalent modifiers and the molten state. We suggest that the most stable titanate glasses form close to compositions where, on average, two oxygen anions bond to each titanium, allowing a continuous, disordered Ti–O network of bridging oxygen (OTi2), or with equal numbers of OTi3 triclusters and OTi1 non–bridging oxygen in charge–balance. Here, we report on new glasses formed from praseodymium, europium, and gadolinium titanate melts, the latter being the smallest rare–earth for which binary titanate glasses have been obtained.
- Authors:
-
- Materials Development, Inc., Arlington Heights, IL (United States)
- Argonne National Lab. (ANL), Argonne, IL (United States). X-Ray Science Div.
- Materials Development, Inc., Arlington Heights, IL (United States); Argonne National Lab. (ANL), Argonne, IL (United States). X-Ray Science Div.
- Publication Date:
- Research Org.:
- Argonne National Laboratory (ANL), Argonne, IL (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Small Business Innovation Research (SBIR) and Small Business Technology Transfer (STTR); USDOE
- OSTI Identifier:
- 1574297
- Alternate Identifier(s):
- OSTI ID: 1560342
- Grant/Contract Number:
- AC02-06CH11357; SC0015241; SC0018601
- Resource Type:
- Accepted Manuscript
- Journal Name:
- International Journal of Applied Glass Science
- Additional Journal Information:
- Journal Volume: 10; Journal Issue: 4; Journal ID: ISSN 2041-1286
- Publisher:
- American Ceramic Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 36 MATERIALS SCIENCE; characterization; glass forming melts; glass forming systems; structure; X‐ray absorption; X‐ray diffraction
Citation Formats
Alderman, Oliver L. G., Benmore, Chris J., Tamalonis, Anthony J., and Weber, Rick. Rare-earth titanate melt structure and glass formation. United States: N. p., 2019.
Web. doi:10.1111/ijag.13479.
Alderman, Oliver L. G., Benmore, Chris J., Tamalonis, Anthony J., & Weber, Rick. Rare-earth titanate melt structure and glass formation. United States. https://doi.org/10.1111/ijag.13479
Alderman, Oliver L. G., Benmore, Chris J., Tamalonis, Anthony J., and Weber, Rick. Wed .
"Rare-earth titanate melt structure and glass formation". United States. https://doi.org/10.1111/ijag.13479. https://www.osti.gov/servlets/purl/1574297.
@article{osti_1574297,
title = {Rare-earth titanate melt structure and glass formation},
author = {Alderman, Oliver L. G. and Benmore, Chris J. and Tamalonis, Anthony J. and Weber, Rick},
abstractNote = {The structure of rare–earth titanate melts and glasses of composition 17RE2O3.83TiO2 have been investigated in situ by aerodynamic levitation with laser heating. Ti K–edge X–ray absorption near–edge structure (XANES) spectroscopy reveals an effect of RE cation size on mean Ti–O coordination numbers (nTiO), which increase from ~4.8(2) in glass–forming La titanate to ~5.1(2) in non–glass–forming Sc titanate liquids. We suggest that the associated increase in OTi3 triclusters in melts bearing smaller RE cations tends to inhibit glass formation. Both XANES and high–energy X–ray diffraction indicate increases in nTiO as the liquids supercool and vitrify. Results are discussed in the context of alkali and alkaline–earth titanate glasses, extending the observed dependence of nTiO on structural basicity (modifier content divided by potential) to trivalent modifiers and the molten state. We suggest that the most stable titanate glasses form close to compositions where, on average, two oxygen anions bond to each titanium, allowing a continuous, disordered Ti–O network of bridging oxygen (OTi2), or with equal numbers of OTi3 triclusters and OTi1 non–bridging oxygen in charge–balance. Here, we report on new glasses formed from praseodymium, europium, and gadolinium titanate melts, the latter being the smallest rare–earth for which binary titanate glasses have been obtained.},
doi = {10.1111/ijag.13479},
journal = {International Journal of Applied Glass Science},
number = 4,
volume = 10,
place = {United States},
year = {Wed Jul 03 00:00:00 EDT 2019},
month = {Wed Jul 03 00:00:00 EDT 2019}
}
Figures / Tables:
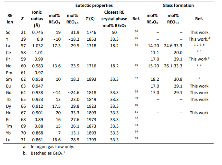 Table 1: Properties of the rare earth ions and rare-earth sesquioxide-titanium dioxide systems. The ionic radii54 are for trivalent cations in 6-fold coordination to oxygen, for sake of comparison. Hyphens (–) indicate lack of any glass formation for the 17RE2O3.83TiO2 compositions tested herein.
Table 1: Properties of the rare earth ions and rare-earth sesquioxide-titanium dioxide systems. The ionic radii54 are for trivalent cations in 6-fold coordination to oxygen, for sake of comparison. Hyphens (–) indicate lack of any glass formation for the 17RE2O3.83TiO2 compositions tested herein.
Works referenced in this record:
ATHENA , ARTEMIS , HEPHAESTUS : data analysis for X-ray absorption spectroscopy using IFEFFIT
journal, June 2005
- Ravel, B.; Newville, M.
- Journal of Synchrotron Radiation, Vol. 12, Issue 4
Infrared refractive index of diamond
journal, January 1981
- Edwards, David F.; Ochoa, Ellen
- Journal of the Optical Society of America, Vol. 71, Issue 5
Refractive index calculation using the structural properties of La4Ti9O24 glass
journal, May 2008
- Arai, Yasutomo; Itoh, Keiji; Kohara, Shinji
- Journal of Applied Physics, Vol. 103, Issue 9
Density of thin TiO2 films
journal, June 1996
- Laube, M.; Rauch, F.; Ottermann, C.
- Nuclear Instruments and Methods in Physics Research Section B: Beam Interactions with Materials and Atoms, Vol. 113, Issue 1-4
Phase relationship in the TiO2–Nd2O3 pseudo-binary system
journal, January 2013
- Gong, Weiping; Zhang, Rui
- Journal of Alloys and Compounds, Vol. 548
Liquid B 2 O 3 up to 1700 K: x-ray diffraction and boroxol ring dissolution
journal, October 2015
- Alderman, O. L. G.; Ferlat, G.; Baroni, A.
- Journal of Physics: Condensed Matter, Vol. 27, Issue 45
Continuous Structural Transition in Glass-Forming Molten Titanate BaTi 2 O 5
journal, November 2016
- Alderman, O. L. G.; Benmore, C. J.; Tamalonis, A.
- The Journal of Physical Chemistry C, Vol. 120, Issue 47
Structure of binary K2OTiO2 and Cs2OTiO2 glasses
journal, October 1989
- Sakka, Sumio; Miyaji, Fumiaki; Fukumi, Kohei
- Journal of Non-Crystalline Solids, Vol. 112, Issue 1-3
Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides
journal, September 1976
- Shannon, R. D.
- Acta Crystallographica Section A, Vol. 32, Issue 5, p. 751-767
Study on upconversion luminescence and thermal properties of Ho3+/Yb3+ co-doped La2O3–TiO2–ZrO2 glasses
journal, July 2016
- Zhang, Minghui; Wen, Haiqin; Yu, Huimei
- Journal of Alloys and Compounds, Vol. 672
Aerodynamic levitator for in situ x-ray structure measurements on high temperature and molten nuclear fuel materials
journal, July 2016
- Weber, J. K. R.; Tamalonis, A.; Benmore, C. J.
- Review of Scientific Instruments, Vol. 87, Issue 7
Glass formation of rare earth aluminates by containerless processing
journal, December 2012
- Watanabe, Yasuhiro; Masuno, Atsunobu; Inoue, Hiroyuki
- Journal of Non-Crystalline Solids, Vol. 358, Issue 24
Natural widths of atomic K and L levels, K α X‐ray lines and several K L L Auger lines
journal, April 1979
- Krause, M. O.; Oliver, J. H.
- Journal of Physical and Chemical Reference Data, Vol. 8, Issue 2
Temperature-Driven Structural Transitions in Molten Sodium Borates Na 2 O–B 2 O 3 : X-ray Diffraction, Thermodynamic Modeling, and Implications for Topological Constraint Theory
journal, December 2015
- Alderman, O. L. G.; Liška, M.; Macháček, J.
- The Journal of Physical Chemistry C, Vol. 120, Issue 1
Borate melt structure: Temperature‐dependent B–O bond lengths and coordination numbers from high‐energy X‐ray diffraction
journal, March 2018
- Alderman, Oliver L. G.; Benmore, Chris J.; Lin, Alex
- Journal of the American Ceramic Society, Vol. 101, Issue 8
Comprehensive Structural Study of Glassy and Metastable Crystalline BaTi 2 O 5
journal, January 2009
- Yu, Jianding; Kohara, Shinji; Itoh, Keiji
- Chemistry of Materials, Vol. 21, Issue 2
Glass Formation in LaO3/2-TiO2 Binary System by Containerless Processing
journal, December 2011
- Kaneko, Masashi; Yu, Jianding; Masuno, Atsunobu
- Journal of the American Ceramic Society, Vol. 95, Issue 1
Optical super-resolution by high-index liquid-immersed microspheres
journal, October 2012
- Darafsheh, Arash; Walsh, Gary F.; Dal Negro, Luca
- Applied Physics Letters, Vol. 101, Issue 14
Laser hearth melt processing of ceramic materials
journal, February 1996
- Richard Weber, J. K.; Felten, J. J.; Nordine, Paul C.
- Review of Scientific Instruments, Vol. 67, Issue 2
Rapid quenching on the binary systems of high temperature oxides
journal, June 1974
- Suzuki, Takeyuki; Anthony, Anne-Marie
- Materials Research Bulletin, Vol. 9, Issue 6
The Density of Titanium(IV) Oxide Liquid
journal, October 1991
- Dingwell, Donald B.
- Journal of the American Ceramic Society, Vol. 74, Issue 10
Structure of molten titanium dioxide
journal, September 2014
- Alderman, O. L. G.; Skinner, L. B.; Benmore, C. J.
- Physical Review B, Vol. 90, Issue 9
Molten barium titanate: a high-pressure liquid silicate analogue
journal, March 2019
- Alderman, O. L. G.; Benmore, C. J.; Neuefeind, J.
- Journal of Physics: Condensed Matter, Vol. 31, Issue 20
Vitreous forsterite (Mg 2 SiO 4 ): Synthesis, structure, and thermochemistry
journal, July 2001
- Tangeman, Jean A.; Phillips, Brian L.; Navrotsky, Alexandra
- Geophysical Research Letters, Vol. 28, Issue 13
Low Cation Coordination in Oxide Melts
journal, April 2014
- Skinner, L. B.; Benmore, C. J.; Weber, J. K. R.
- Physical Review Letters, Vol. 112, Issue 15
Influence of the Atmosphere on the Oxidation State of the Eu-Ion in a SiAIO(N) Glass and Glass-Ceramic
journal, January 2000
- Menke, Yvonne; Baron, V.; Lemercier, H.
- Materials Science Forum, Vol. 325-326
Local structural variation with oxygen fugacity in Fe2SiO4+ fayalitic iron silicate melts
journal, April 2017
- Alderman, O. L. G.; Lazareva, L.; Wilding, M. C.
- Geochimica et Cosmochimica Acta, Vol. 203
Glass-forming region and high refractive index of TiO 2 -based glasses prepared by containerless processing : Glass-forming region and high refractive index of TiO
journal, October 2012
- Masuno, Atsunobu; Watanabe, Yasuhiro; Inoue, Hiroyuki
- physica status solidi (c), Vol. 9, Issue 12
Investigation of TiO2SiO2 glasses by X-ray absorption spectroscopy
journal, April 1983
- Greegor, Robert B.; Lytle, Farrel W.; Sandstrom, Donald R.
- Journal of Non-Crystalline Solids, Vol. 55, Issue 1
Refractive index dispersion, optical transmittance, and Raman scattering of BaTi2O5 glass
journal, September 2010
- Masuno, A.; Inoue, H.; Yu, J.
- Journal of Applied Physics, Vol. 108, Issue 6
Measurements of liquid and glass structures using aerodynamic levitation and in-situ high energy x-ray and neutron scattering
journal, January 2014
- Weber, J. K. R.; Benmore, C. J.; Skinner, L. B.
- Journal of Non-Crystalline Solids, Vol. 383
Phase Relations in the System Y2O3-TiO2
journal, March 1976
- Mizutani, Nobuyasu; Tajima, Yo; Kato, Masanori
- Journal of the American Ceramic Society, Vol. 59, Issue 3-4
Structure of Na2O�2TiO2 glass
journal, January 1991
- Miyaji, F.; Yoko, T.; Kozuka, H.
- Journal of Materials Science, Vol. 26, Issue 1
Phase stability and equilibria in the La2O3–TiO2 system
journal, July 2000
- Škapin, Srečo D.; Kolar✠, Drago; Suvorov, Danilo
- Journal of the European Ceramic Society, Vol. 20, Issue 8
Study on the structure of K2O·2TiO2 glass by X-ray radial distribution analysis
journal, January 1989
- Sakka, S.; Miyaji, F.; Fukumi, K.
- Journal of Non-Crystalline Solids, Vol. 107, Issue 2-3
Synthesis of amorphous La 4 Ti 9 O 24 microspheres with high-refractive index via containerless flame-spraying method
journal, January 2018
- Li, Xiaoyu; Ma, Xiaoguang; Li, Jiangtao
- Materials Research Bulletin, Vol. 97
Solidification and thermophysical property studies of barium titanate using electrostatic levitation furnace
journal, July 2006
- Yu, Jianding; Ishikawa, Takehiko; Paradis, Paul-François
- Journal of Crystal Growth, Vol. 292, Issue 2
Spectral Absorption Coefficient of Molten Aluminum Oxide from.0.385 to 0.780 mum
journal, March 1995
- Weber, J. K. Richard; Krishnan, Shankar; Anderson, Collin D.
- Journal of the American Ceramic Society, Vol. 78, Issue 3
Phase equilibrium relationships in the system Gd2O3-TiO2
journal, May 1965
- Waring, J. L.; Schneider, S. J.
- Journal of Research of the National Bureau of Standards Section A: Physics and Chemistry, Vol. 69A, Issue 3
The Structural Role of Lanthanum and Yttrium in Aluminosilicate Glasses: A 27 Al and 17 O MAS NMR Study
journal, December 1998
- Schaller, Torsten; Stebbins, Jonathan F.
- The Journal of Physical Chemistry B, Vol. 102, Issue 52
Bright white upconversion luminescence from Er3+/Tm3+/Yb3+-doped titanate-based glasses prepared by aerodynamic levitation method
journal, October 2017
- Zhang, Minghui; Yu, Jianding; Jiang, Wan
- Optical Materials, Vol. 72
Iron K-edge X-ray absorption near-edge structure spectroscopy of aerodynamically levitated silicate melts and glasses
journal, March 2017
- Alderman, O. L. G.; Wilding, M. C.; Tamalonis, A.
- Chemical Geology, Vol. 453
Density and refractive index of TiO2 films prepared by reactive evaporation
journal, August 2000
- Mergel, D.; Buschendorf, D.; Eggert, S.
- Thin Solid Films, Vol. 371, Issue 1-2
The partial molar volume and thermal expansivity of TiO 2 in alkali silicate melts: Systematic variation with Ti coordination
journal, July 2001
- Liu, Qiong; Lange, Rebecca A.
- Geochimica et Cosmochimica Acta, Vol. 65, Issue 14
Preparation and properties of binary oxide glasses containing rare earth oxides. [希土類含有高融点酸化物ガラスの作製とその二,三の物性]
journal, January 1986
- Kozuka, Hiromitsu; Ota, Rikuo; Soga, Naohiro
- Journal of the Society of Materials Science, Japan, Vol. 35, Issue 388
Ti -edge XANES studies of Ti coordination and disorder in oxide compounds: Comparison between theory and experiment
journal, July 1997
- Farges, François; Brown, Gordon E.; Rehr, J. J.
- Physical Review B, Vol. 56, Issue 4
Surface Tensions and Densities of Melts in TiO<SUB>2</SUB>-BaO and TiO<SUB>2</SUB>-Na<SUB>2</SUB>O Systems [TiO2-BaO系融体およびTiO2-Na2O系融体の表面張力と密度]
journal, January 1993
- Ikemiya, Norihito; Yoshitomi, Jun; Hara, Shigeta
- Journal of the Japan Institute of Metals, Vol. 57, Issue 5
Effect of atomic vibrations on the x-ray absorption spectra at the edge of Al in and of Ti in rutile
journal, March 2010
- Brouder, Christian; Cabaret, Delphine; Juhin, Amélie
- Physical Review B, Vol. 81, Issue 11
Borate melt structure: a short review
journal, February 2018
- Alderman, Oliver
- Physics and Chemistry of Glasses: European Journal of Glass Science and Technology Part B, Vol. 59, Issue 1
IFEFFIT : interactive XAFS analysis and FEFF fitting
journal, March 2001
- Newville, Matthew
- Journal of Synchrotron Radiation, Vol. 8, Issue 2
An interpretation of glass chemistry in terms of the optical basicity concept
journal, August 1976
- Duffy, J. A.; Ingram, M. D.
- Journal of Non-Crystalline Solids, Vol. 21, Issue 3
Magnetosomes could be protective shields against metal stress in magnetotactic bacteria
journal, July 2020
- Muñoz, D.; Marcano, L.; Martín-Rodríguez, R.
- Scientific Reports, Vol. 10, Issue 1
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal