Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting
Abstract
Hydrogen production from photoelectrochemical (PEC) water splitting using semiconductor photocatalysts has attracted great attention to realize clean and renewable energy from solar energy. The visible light response of WO3 with a long hole diffusion length (~150 nm) and good electron mobility (~12 cm2 V–1 s–1) makes it suitable as the photoanode. Yet, WO3 suffers from issues including rapid recombination of photoexcited electron–hole pairs, photo-corrosion during the photocatalytic process due to the formation of peroxo-species, sluggish kinetics of photogenerated holes, and slow charge transfer at the semiconductor/electrolyte interface. Our report highlights the approaches to overcome these drawbacks of WO3 photoanodes, including: (i) the manipulation of nanostructured WO3 photoanodes to decrease the nanoparticle size to promote hole migration to the WO3/electrolyte interface which benefits the charge separation; (ii) doping or introducing oxygen vacancies to improve electrical conductivity; exposing high energy crystal surfaces to promote the consumption of photogenerated holes on the high-active crystal face, thereby suppressing the recombination of photogenerated electrons and holes; (iii) decorating with co-catalysts to reduce the overpotential which inhibits the formation of peroxo-species; (iv) other methods such as coupling with narrow band semiconductors to accelerate the charge separation and controlling the crystal phase via annealing to reduce defects.more »
- Authors:
-
- Beijing Univ. of Technology (China); Univ. of Tennessee, Knoxville, TN (United States)
- Beijing Univ. of Technology (China)
- Zhengzhou Univ. (China)
- Univ. of Tennessee, Knoxville, TN (United States); Pondicherry Univ. (India)
- Henan Univ., Kaifeng (China)
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Univ. of Tennessee, Knoxville, TN (United States)
- Publication Date:
- Research Org.:
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); Beijing Natural Science Foundation; National Natural Science Foundation
- OSTI Identifier:
- 1546539
- Alternate Identifier(s):
- OSTI ID: 1559057
- Grant/Contract Number:
- AC05-00OR22725
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Nanoscale
- Additional Journal Information:
- Journal Volume: 2019; Journal Issue: 11; Journal ID: ISSN 2040-3364
- Publisher:
- Royal Society of Chemistry
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 29 ENERGY PLANNING, POLICY, AND ECONOMY; 08 HYDROGEN
Citation Formats
Zheng, Guangwei, Wang, Jinshu, Liu, Hu, Murugadoss, Vignesh, Zu, Guannan, Che, Haibing, Lai, Chen, Li, Hongyi, Ding, Tao, Gao, Qiang, and Guo, Zhanhu. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. United States: N. p., 2019.
Web. doi:10.1039/C9NR03474A.
Zheng, Guangwei, Wang, Jinshu, Liu, Hu, Murugadoss, Vignesh, Zu, Guannan, Che, Haibing, Lai, Chen, Li, Hongyi, Ding, Tao, Gao, Qiang, & Guo, Zhanhu. Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting. United States. https://doi.org/10.1039/C9NR03474A
Zheng, Guangwei, Wang, Jinshu, Liu, Hu, Murugadoss, Vignesh, Zu, Guannan, Che, Haibing, Lai, Chen, Li, Hongyi, Ding, Tao, Gao, Qiang, and Guo, Zhanhu. Fri .
"Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting". United States. https://doi.org/10.1039/C9NR03474A. https://www.osti.gov/servlets/purl/1546539.
@article{osti_1546539,
title = {Tungsten oxide nanostructures and nanocomposites for photoelectrochemical water splitting},
author = {Zheng, Guangwei and Wang, Jinshu and Liu, Hu and Murugadoss, Vignesh and Zu, Guannan and Che, Haibing and Lai, Chen and Li, Hongyi and Ding, Tao and Gao, Qiang and Guo, Zhanhu},
abstractNote = {Hydrogen production from photoelectrochemical (PEC) water splitting using semiconductor photocatalysts has attracted great attention to realize clean and renewable energy from solar energy. The visible light response of WO3 with a long hole diffusion length (~150 nm) and good electron mobility (~12 cm2 V–1 s–1) makes it suitable as the photoanode. Yet, WO3 suffers from issues including rapid recombination of photoexcited electron–hole pairs, photo-corrosion during the photocatalytic process due to the formation of peroxo-species, sluggish kinetics of photogenerated holes, and slow charge transfer at the semiconductor/electrolyte interface. Our report highlights the approaches to overcome these drawbacks of WO3 photoanodes, including: (i) the manipulation of nanostructured WO3 photoanodes to decrease the nanoparticle size to promote hole migration to the WO3/electrolyte interface which benefits the charge separation; (ii) doping or introducing oxygen vacancies to improve electrical conductivity; exposing high energy crystal surfaces to promote the consumption of photogenerated holes on the high-active crystal face, thereby suppressing the recombination of photogenerated electrons and holes; (iii) decorating with co-catalysts to reduce the overpotential which inhibits the formation of peroxo-species; (iv) other methods such as coupling with narrow band semiconductors to accelerate the charge separation and controlling the crystal phase via annealing to reduce defects. These methods are reviewed with detailed examples.},
doi = {10.1039/C9NR03474A},
journal = {Nanoscale},
number = 11,
volume = 2019,
place = {United States},
year = {Fri Jun 28 00:00:00 EDT 2019},
month = {Fri Jun 28 00:00:00 EDT 2019}
}
Web of Science
Figures / Tables:
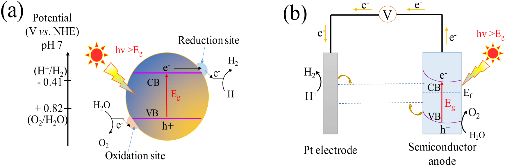 Fig. 1: Schematic illustration of (a) photocatalytic water splitting for a one-step photoexcitation system, (b) The basic principles of water splitting for a PEC cell with an n-type semiconductor photoanode and Pt cathode. CB, conduction band; VB, valence band; Eg, band gap; Ef, fermi level.
Fig. 1: Schematic illustration of (a) photocatalytic water splitting for a one-step photoexcitation system, (b) The basic principles of water splitting for a PEC cell with an n-type semiconductor photoanode and Pt cathode. CB, conduction band; VB, valence band; Eg, band gap; Ef, fermi level.
Works referenced in this record:
The graphene/lanthanum oxide nanocomposites as electrode materials of supercapacitors
journal, April 2019
- Zhang, Jiaoxia; Zhang, Zhuangzhuang; Jiao, Yueting
- Journal of Power Sources, Vol. 419
Iridium‐Based Catalysts for Solid Polymer Electrolyte Electrocatalytic Water Splitting
journal, April 2019
- Wang, Chao; Lan, Feifei; He, Zhenfeng
- ChemSusChem, Vol. 12, Issue 8
Progress on the Photocatalytic Reduction Removal of Chromium Contamination
journal, November 2018
- Zhao, Zengying; An, He; Lin, Jing
- The Chemical Record, Vol. 19, Issue 5
Bio-template synthesized NiO/C hollow microspheres with enhanced Li-ion battery electrochemical performance
journal, January 2018
- Tian, Jiangyang; Shao, Qian; Dong, Xiaojie
- Electrochimica Acta, Vol. 261
Recent theoretical progress in the development of photoanode materials for solar water splitting photoelectrochemical cells
journal, January 2015
- Bhatt, Mahesh Datt; Lee, Jae Sung
- Journal of Materials Chemistry A, Vol. 3, Issue 20
Insight into Charge Separation in WO 3 /BiVO 4 Heterojunction for Solar Water Splitting
journal, August 2016
- Chae, Sang Youn; Lee, Chang Soo; Jung, Hyejin
- ACS Applied Materials & Interfaces, Vol. 9, Issue 23
Crystal Structure Modification Enhanced FeNb 11 O 29 Anodes for Lithium-Ion Batteries
journal, September 2017
- Lou, Xiaoming; Lin, Chunfu; Luo, Qiang
- ChemElectroChem, Vol. 4, Issue 12
All-Solid-State Z-Scheme Photocatalytic Systems
journal, May 2014
- Zhou, Peng; Yu, Jiaguo; Jaroniec, Mietek
- Advanced Materials, Vol. 26, Issue 29
A significant cathodic shift in the onset potential and enhanced photoelectrochemical water splitting using Au nanoparticles decorated WO3 nanorod array
journal, November 2015
- Xu, Fang; Yao, Yanwen; Bai, Dandan
- Journal of Colloid and Interface Science, Vol. 458
Roles of Cocatalysts in Photocatalysis and Photoelectrocatalysis
journal, March 2013
- Yang, Jinhui; Wang, Donge; Han, Hongxian
- Accounts of Chemical Research, Vol. 46, Issue 8
Achieving carbon-rich silicon-containing ceramic anode for advanced lithium ion battery
journal, June 2019
- Idrees, Muhammad; Batool, Saima; Zhuang, Qiang
- Ceramics International, Vol. 45, Issue 8
Oxygen vacancy derived local build-in electric field in mesoporous hollow Co 3 O 4 microspheres promotes high-performance Li-ion batteries
journal, January 2018
- Hou, Chuanxin; Hou, Yue; Fan, Yuqi
- Journal of Materials Chemistry A, Vol. 6, Issue 16
Hydrothermal synthesis and photoelectrochemical properties of vertically aligned tungsten trioxide (hydrate) plate-like arrays fabricated directly on FTO substrates
journal, January 2012
- Yang, Jiao; Li, Wenzhang; Li, Jie
- Journal of Materials Chemistry, Vol. 22, Issue 34
Direct Z-scheme g-C3N4/WO3 photocatalyst with atomically defined junction for H2 production
journal, December 2017
- Yu, Weilai; Chen, Junxiang; Shang, Tongtong
- Applied Catalysis B: Environmental, Vol. 219
Limiting factors for photochemical charge separation in BiVO 4 /Co 3 O 4 , a highly active photocatalyst for water oxidation in sunlight
journal, January 2014
- Wang, Jiarui; Osterloh, Frank E.
- J. Mater. Chem. A, Vol. 2, Issue 24
RuO 2 -Loaded β-Ge 3 N 4 as a Non-Oxide Photocatalyst for Overall Water Splitting
journal, March 2005
- Sato, Junya; Saito, Nobuo; Yamada, Yoko
- Journal of the American Chemical Society, Vol. 127, Issue 12
Dual Oxygen and Tungsten Vacancies on a WO 3 Photoanode for Enhanced Water Oxidation
journal, August 2016
- Ma, Ming; Zhang, Kan; Li, Ping
- Angewandte Chemie International Edition, Vol. 55, Issue 39
Epitaxial growth of WO 3 nanoneedles achieved using a facile flame surface treatment process engineering of hole transport and water oxidation reactivity
journal, January 2018
- Shi, Xinjian; Cai, Lili; Choi, Il Yong
- Journal of Materials Chemistry A, Vol. 6, Issue 40
Improving Visible-light Responses and Electric Conductivities by Incorporating Sb2S3 and Reduced Graphene Oxide in a WO3 Nanoplate Array for Photoelectrochemical Water Oxidation
journal, October 2017
- Lin, Hong-Syun; Lin, Lu-Yin
- Electrochimica Acta, Vol. 252
Quasi-core-shell TiO2/WO3 and WO3/TiO2 nanorod arrays fabricated by glancing angle deposition for solar water splitting
journal, January 2011
- Smith, Wilson; Wolcott, Abraham; Fitzmorris, Robert Carl
- Journal of Materials Chemistry, Vol. 21, Issue 29
Exfoliated thin Bi2MoO6 nanosheets supported on WO3 electrode for enhanced photoelectrochemical water splitting
journal, December 2016
- Ma, Ying; Jia, Yulong; Wang, Lina
- Applied Surface Science, Vol. 390
Nitrogen vacancy engineered graphitic C3N4-based polymers for photocatalytic oxidation of aromatic alcohols to aldehydes
journal, February 2018
- Ding, Jing; Xu, Wei; Wan, Hui
- Applied Catalysis B: Environmental, Vol. 221
Sandwich-like NiCo layered double hydroxide/reduced graphene oxide nanocomposite cathodes for high energy density asymmetric supercapacitors
journal, January 2019
- Le, Kai; Wang, Zhou; Wang, Fenglong
- Dalton Transactions, Vol. 48, Issue 16
In Situ Formation of an Oxygen-Evolving Catalyst in Neutral Water Containing Phosphate and Co2+
journal, August 2008
- Kanan, M. W.; Nocera, D. G.
- Science, Vol. 321, Issue 5892, p. 1072-1075
Electrochemical Doping Induced In Situ Homo-species for Enhanced Photoelectrochemical Performance on WO3 Nanoparticles Film Photoelectrodes
journal, August 2016
- Liu, Yang; Li, Jie; Li, Wenzhang
- Electrochimica Acta, Vol. 210
Nanosheet-based Nb 12 O 29 hierarchical microspheres for enhanced lithium storage
journal, January 2019
- Li, Renjie; Zhu, Xiangzhen; Fu, Qingfeng
- Chemical Communications, Vol. 55, Issue 17
Facile Preparation of 1T/2H‐Mo(S 1‐x Se x ) 2 Nanoparticles for Boosting Hydrogen Evolution Reaction
journal, March 2019
- Lin, Zhiping; Lin, Bo; Wang, Zongpeng
- ChemCatChem, Vol. 11, Issue 8
Doping of WO 3 for Photocatalytic Water Splitting: Hints from Density Functional Theory
journal, April 2012
- Wang, Fenggong; Di Valentin, Cristiana; Pacchioni, Gianfranco
- The Journal of Physical Chemistry C, Vol. 116, Issue 16
Nano-TiNb2O7/carbon nanotubes composite anode for enhanced lithium-ion storage
journal, January 2018
- Lin, Chunfu; Hu, Lei; Cheng, Chuanbing
- Electrochimica Acta, Vol. 260
An overview of metamaterials and their achievements in wireless power transfer
journal, January 2018
- Sun, Kai; Fan, Runhua; Zhang, Xihua
- Journal of Materials Chemistry C, Vol. 6, Issue 12
Photocatalytic oxidation of water by polymeric carbon nitride nanohybrids made of sustainable elements
journal, January 2012
- Zhang, Jinshui; Grzelczak, Marek; Hou, Yidong
- Chem. Sci., Vol. 3, Issue 2
NH4-doped anodic WO3 prepared through anodization and subsequent NH4OH treatment for water splitting
journal, January 2015
- Choi, Yong-Wook; Kim, Sunkyu; Seong, Mijeong
- Applied Surface Science, Vol. 324
Large Scaled Synthesis of Heterostructured Electrospun TiO 2 /SnO 2 Nanofibers with an Enhanced Photocatalytic Activity
journal, January 2017
- Zhang, Li; Yu, Wei; Han, Cui
- Journal of The Electrochemical Society, Vol. 164, Issue 9
Conformally coated BiVO4 nanodots on porosity-controlled WO3 nanorods as highly efficient type II heterojunction photoanodes for water oxidation
journal, October 2016
- Lee, Mi Gyoung; Kim, Do Hong; Sohn, Woonbae
- Nano Energy, Vol. 28
Growth of Oriented Single-Crystalline Rutile TiO 2 Nanorods on Transparent Conducting Substrates for Dye-Sensitized Solar Cells
journal, March 2009
- Liu, Bin; Aydil, Eray S.
- Journal of the American Chemical Society, Vol. 131, Issue 11
An efficient binder-free electrode with multiple carbonized channels wrapped by NiCo2O4 nanosheets for high-performance capacitive energy storage
journal, January 2019
- Qu, Zhichao; Shi, Minjie; Wu, Hanzhao
- Journal of Power Sources, Vol. 410-411
Enhanced photoelectrochemical performance of plate-like WO 3 induced by surface oxygen vacancies
journal, July 2016
- Liu, Yang; Li, Jie; Tang, Hui
- Electrochemistry Communications, Vol. 68
Enhancement of photoelectrochemical water splitting response of WO3 by Means of Bi doping
journal, January 2018
- Kalanur, Shankara S.; Yoo, Il-Han; Eom, Kiryung
- Journal of Catalysis, Vol. 357
Large-Scale Tunable 3D Self-Supporting WO 3 Micro-Nano Architectures as Direct Photoanodes for Efficient Photoelectrochemical Water Splitting
journal, May 2017
- Cai, Mingyong; Fan, Peixun; Long, Jiangyou
- ACS Applied Materials & Interfaces, Vol. 9, Issue 21
Electrochemical synthesis of CdS/ZnO nanotube arrays with excellent photoelectrochemical properties
journal, January 2012
- Qi, Xiaopeng; She, Guangwei; Liu, Yunyu
- Chem. Commun., Vol. 48, Issue 2
Facile synthesis of Ni-doped WO3 nanoplate arrays for effective photoelectrochemical water splitting
journal, July 2017
- Xiao, Yong-Hao; Xu, Cheng-Qun; Zhang, Wei-De
- Journal of Solid State Electrochemistry, Vol. 21, Issue 11
Analysis of charge separation processes in WO 3 -BiVO 4 composite for efficient photoelectrochemical water oxidation
journal, March 2017
- Seo, Jong Hyeok; Park, Gisang; Oh, Kyung Hee
- Journal of Electroanalytical Chemistry, Vol. 789
Quantitative Analysis and Visualized Evidence for High Charge Separation Efficiency in a Solid-Liquid Bulk Heterojunction
journal, March 2014
- Zhao, Xin; Luo, Wenjun; Feng, Jianyong
- Advanced Energy Materials, Vol. 4, Issue 9
Potassium Hydroxide Activated and Nitrogen Doped Graphene with Enhanced Supercapacitive Behavior
journal, July 2018
- Deng, Weiyuan; Kang, Tianhe; Liu, Hu
- Science of Advanced Materials, Vol. 10, Issue 7
TiO 2 films with oriented anatase {001} facets and their photoelectrochemical behavior as CdS nanoparticle sensitized photoanodes
journal, January 2011
- Wang, Xuewen; Liu, Gang; Wang, Lianzhou
- J. Mater. Chem., Vol. 21, Issue 3
Direct Z-scheme TiO2/CdS hierarchical photocatalyst for enhanced photocatalytic H2-production activity
journal, November 2017
- Meng, Aiyun; Zhu, Bicheng; Zhong, Bo
- Applied Surface Science, Vol. 422
Enhanced Charge-Collection Efficiencies and Light Scattering in Dye-Sensitized Solar Cells Using Oriented TiO 2 Nanotubes Arrays
journal, January 2007
- Zhu, Kai; Neale, Nathan R.; Miedaner, Alexander
- Nano Letters, Vol. 7, Issue 1
Elegant Z-scheme-dictated g-C 3 N 4 enwrapped WO 3 superstructures: a multifarious platform for versatile photoredox catalysis
journal, January 2017
- Liang, Zhi-Yu; Wei, Jin-Xin; Wang, Xiu
- Journal of Materials Chemistry A, Vol. 5, Issue 30
Assembly of g-C 3 N 4 -based type II and Z-scheme heterojunction anodes with improved charge separation for photoelectrojunction water oxidation
journal, January 2017
- Wang, Cai-He; Qin, Dong-Dong; Shan, Duo-Liang
- Physical Chemistry Chemical Physics, Vol. 19, Issue 6
One-pot synthesized molybdenum dioxide–molybdenum carbide heterostructures coupled with 3D holey carbon nanosheets for highly efficient and ultrastable cycling lithium-ion storage
journal, January 2019
- Hou, Chuanxin; Wang, Jun; Du, Wei
- Journal of Materials Chemistry A, Vol. 7, Issue 22
A hybrid carbon aerogel with both aligned and interconnected pores as interlayer for high-performance lithium–sulfur batteries
journal, September 2016
- Liu, Mingkai; Yang, Zhibin; Sun, Hao
- Nano Research, Vol. 9, Issue 12
Brain-Like Navigation Scheme based on MEMS-INS and Place Recognition
journal, April 2019
- Shen, Chong; Liu, Xiaochen; Cao, Huiliang
- Applied Sciences, Vol. 9, Issue 8
Efficient solid-state perovskite solar cells based on nanostructured zinc oxide designed by strategic low temperature water oxidation
journal, January 2017
- Pelicano, Christian Mark; Yanagi, Hisao
- Journal of Materials Chemistry C, Vol. 5, Issue 32
Zinc oxide/vanadium pentoxide heterostructures with enhanced day-night antibacterial activities
journal, July 2019
- Sun, Haiyun; Yang, Zhaoqing; Pu, Yanan
- Journal of Colloid and Interface Science, Vol. 547
Photoelectrochemical Property of Tungsten Oxide Films of Vertically Aligned Flakes for Visible-Light-Induced Water Oxidation
journal, January 2011
- Amano, Fumiaki; Li, Ding; Ohtani, Bunsho
- Journal of The Electrochemical Society, Vol. 158, Issue 2
Preparation and water-splitting photocatalytic behavior of S-doped WO3
journal, December 2012
- Li, Wenzhang; Li, Jie; Wang, Xuan
- Applied Surface Science, Vol. 263
A new method for the fabrication of a bilayer WO3/Fe2O3 photoelectrode for enhanced photoelectrochemical performance
journal, February 2018
- Ng, Kim Hang; Minggu, Lorna Jeffery; Mark-Lee, Wun Fui
- Materials Research Bulletin, Vol. 98
Direct growth and photoelectrochemical properties of tungsten oxide nanobelt arrays
journal, January 2008
- Wang, Hua; Quan, Xie; Zhang, Yaobin
- Nanotechnology, Vol. 19, Issue 6
Ti(iv) doped WO3 nanocuboids: fabrication and enhanced visible-light-driven photocatalytic performance
journal, January 2011
- Feng, Chengxin; Wang, Shaozhen; Geng, Baoyou
- Nanoscale, Vol. 3, Issue 9
Enhancing photoelectrochemical water splitting by aluminum-doped plate-like WO3 electrodes
journal, April 2015
- Li, Wenzhang; Zhan, Faqi; Li, Jie
- Electrochimica Acta, Vol. 160
WO 3 photoanodes with controllable bulk and surface oxygen vacancies for photoelectrochemical water oxidation
journal, January 2018
- Zhang, Jijie; Chang, Xiaoxia; Li, Chengcheng
- Journal of Materials Chemistry A, Vol. 6, Issue 8
Template-engineered epitaxial BiVO 4 photoanodes for efficient solar water splitting
journal, January 2017
- Song, Jaesun; Cha, Jaeseong; Lee, Mi Gyoung
- Journal of Materials Chemistry A, Vol. 5, Issue 35
Photocatalytic Water Oxidation with Nonsensitized IrO 2 Nanocrystals under Visible and UV Light
journal, May 2011
- Frame, F. Andrew; Townsend, Troy K.; Chamousis, Rachel L.
- Journal of the American Chemical Society, Vol. 133, Issue 19
Fabrication of CoTiO 3 /g-C 3 N 4 Hybrid Photocatalysts with Enhanced H 2 Evolution: Z-Scheme Photocatalytic Mechanism Insight
journal, May 2016
- Ye, RongQin; Fang, HuaBin; Zheng, Yan-Zhen
- ACS Applied Materials & Interfaces, Vol. 8, Issue 22
Facile structure design based on C 3 N 4 for mediator-free Z-scheme water splitting under visible light
journal, January 2015
- Zhao, Guixia; Huang, Xiubing; Fina, Federica
- Catalysis Science & Technology, Vol. 5, Issue 6
Solution-Processed Two-Dimensional Metal Dichalcogenide-Based Nanomaterials for Energy Storage and Conversion
journal, April 2016
- Cao, Xiehong; Tan, Chaoliang; Zhang, Xiao
- Advanced Materials, Vol. 28, Issue 29
Branched TiO 2 Nanorods for Photoelectrochemical Hydrogen Production
journal, November 2011
- Cho, In Sun; Chen, Zhebo; Forman, Arnold J.
- Nano Letters, Vol. 11, Issue 11
Facile synthesis of tungsten oxide – Bismuth vanadate nanoflakes as photoanode material for solar water splitting
journal, February 2017
- Ibrahim, Akram A. M.; Khan, Ibrahim; Iqbal, Naseer
- International Journal of Hydrogen Energy, Vol. 42, Issue 5
Hydrogen-treated WO3 nanoflakes show enhanced photostability
journal, January 2012
- Wang, Gongming; Ling, Yichuan; Wang, Hanyu
- Energy & Environmental Science, Vol. 5, Issue 3
Facile Green Synthesis of WO 3 ·H 2 O Nanoplates and WO 3 Nanowires with Enhanced Photoelectrochemical Performance
journal, August 2017
- Nayak, Arpan Kumar; Sohn, Youngku; Pradhan, Debabrata
- Crystal Growth & Design, Vol. 17, Issue 9
Three-Dimensional WO 3 Nanoplate/Bi 2 S 3 Nanorod Heterojunction as a Highly Efficient Photoanode for Improved Photoelectrochemical Water Splitting
journal, November 2017
- Wang, Yidan; Tian, Wei; Chen, Liang
- ACS Applied Materials & Interfaces, Vol. 9, Issue 46
Enhanced Visible Light Conversion Efficiency Using Nanocrystalline WO3 Films
journal, April 2001
- Santato, C.; Ulmann, M.; Augustynski, J.
- Advanced Materials, Vol. 13, Issue 7
Facile Solvothermal Method for Fabricating Arrays of Vertically Oriented α-Fe 2 O 3 Nanowires and Their Application in Photoelectrochemical Water Oxidation
journal, November 2011
- Qin, Dong-Dong; Tao, Chun-Lan; In, Su-il
- Energy & Fuels, Vol. 25, Issue 11
Layered double hydroxide modified WO3 nanorod arrays for enhanced photoelectrochemical water splitting
journal, November 2016
- Fan, Xiaoli; Gao, Bin; Wang, Tao
- Applied Catalysis A: General, Vol. 528
Cobalt Phosphate–ZnO Composite Photocatalysts for Oxygen Evolution from Photocatalytic Water Oxidation
journal, April 2012
- Wang, Yabo; Wang, Yongsheng; Jiang, Rongrong
- Industrial & Engineering Chemistry Research, Vol. 51, Issue 30
Highly efficient direct Z-scheme WO3/CdS-diethylenetriamine photocatalyst and its enhanced photocatalytic H2 evolution under visible light irradiation
journal, June 2018
- Hu, Taiping; Li, Pengfei; Zhang, Jinfeng
- Applied Surface Science, Vol. 442
Preparation of hybrid WO3–TiO2 nanotube photoelectrodes using anodization and wet impregnation: Improved water-splitting hydrogen generation performance
journal, February 2013
- Lai, Chin Wei; Sreekantan, Srimala
- International Journal of Hydrogen Energy, Vol. 38, Issue 5
Microwave solvothermal carboxymethyl chitosan templated synthesis of TiO2/ZrO2 composites toward enhanced photocatalytic degradation of Rhodamine B
journal, April 2019
- Tian, Jiangyang; Shao, Qian; Zhao, Junkai
- Journal of Colloid and Interface Science, Vol. 541
Crystallographically Oriented Mesoporous WO3 Films: Synthesis, Characterization, and Applications
journal, October 2001
- Santato, Clara; Odziemkowski, Marek; Ulmann, Martine
- Journal of the American Chemical Society, Vol. 123, Issue 43, p. 10639-10649
Insights into the electronic bands of WO 3 /BiVO 4 /TiO 2 , revealing high solar water splitting efficiency
journal, January 2017
- Kalanur, Shankara S.; Yoo, Il-Han; Park, Jucheol
- Journal of Materials Chemistry A, Vol. 5, Issue 4
Synthesis of WO3/BiVO4 photoanode using a reaction of bismuth nitrate with peroxovanadate on WO3 film for efficient photoelectrocatalytic water splitting and organic pollutant degradation
journal, November 2017
- Zeng, Qingyi; Li, Jinhua; Li, Linsen
- Applied Catalysis B: Environmental, Vol. 217
Electrospun titania nanofibers segregated by graphene oxide for improved visible light photocatalysis
journal, February 2017
- Zhang, Li; Zhang, Qinghong; Xie, Hongyong
- Applied Catalysis B: Environmental, Vol. 201
Z-scheme photocatalytic hydrogen production over WO 3 /g-C 3 N 4 composite photocatalysts
journal, January 2014
- Katsumata, Hideyuki; Tachi, Yusuke; Suzuki, Tohru
- RSC Adv., Vol. 4, Issue 41
Back Electron–Hole Recombination in Hematite Photoanodes for Water Splitting
journal, January 2014
- Le Formal, Florian; Pendlebury, Stephanie R.; Cornuz, Maurin
- Journal of the American Chemical Society, Vol. 136, Issue 6
Effective silicon nanowire arrays/WO 3 core/shell photoelectrode for neutral pH water splitting
journal, June 2017
- Chen, Zhen; Ning, Minghui; Ma, Ge
- Nanotechnology, Vol. 28, Issue 27
Nonaqueous Production of Nanostructured Anatase with High-Energy Facets
journal, December 2008
- Wu, Binghui; Guo, Changyou; Zheng, Nanfeng
- Journal of the American Chemical Society, Vol. 130, Issue 51
Efficient photocatalytic activity of water oxidation over WO3/BiVO4 composite under visible light irradiation
journal, January 2009
- Chatchai, Ponchio; Murakami, Yoshinori; Kishioka, Shin-ya
- Electrochimica Acta, Vol. 54, Issue 3
Recent advances in semiconductors for photocatalytic and photoelectrochemical water splitting
journal, January 2014
- Hisatomi, Takashi; Kubota, Jun; Domen, Kazunari
- Chem. Soc. Rev., Vol. 43, Issue 22
Optimizing the Activity of Nanoneedle Structured WO 3 Photoanodes for Solar Water Splitting: Direct Synthesis via Chemical Vapor Deposition
journal, March 2017
- Kafizas, Andreas; Francàs, Laia; Sotelo-Vazquez, Carlos
- The Journal of Physical Chemistry C, Vol. 121, Issue 11
Hydroxide ions transportation in polynorbornene anion exchange membrane
journal, February 2018
- Wang, Chao; Mo, Biming; He, Zhenfeng
- Polymer, Vol. 138
A highly conductive carbon–sulfur film with interconnected mesopores as an advanced cathode for lithium–sulfur batteries
journal, January 2017
- Liu, Mingkai; Liu, Yuqing; Yan, Yan
- Chemical Communications, Vol. 53, Issue 65
Efficient hydrogen evolution catalysis using ternary pyrite-type cobalt phosphosulphide
journal, September 2015
- Cabán-Acevedo, Miguel; Stone, Michael L.; Schmidt, J. R.
- Nature Materials, Vol. 14, Issue 12
Identifying champion nanostructures for solar water-splitting
journal, July 2013
- Warren, Scott C.; Voïtchovsky, Kislon; Dotan, Hen
- Nature Materials, Vol. 12, Issue 9
Synthesis of WO3 nanoparticles for photocatalytic O2 evolution by thermal decomposition of ammonium tungstate loading on g-C3N4
journal, June 2011
- Yan, Hongjian; Zhang, Xiaojun; Zhou, Shiqing
- Journal of Alloys and Compounds, Vol. 509, Issue 24
Construction of novel WO3/SnNb2O6 hybrid nanosheet heterojunctions as efficient Z-scheme photocatalysts for pollutant degradation
journal, November 2017
- Ma, Xiaodong; Ma, Wanxia; Jiang, Deli
- Journal of Colloid and Interface Science, Vol. 506
WO3 nanocrystals with tunable percentage of (001)-facet exposure
journal, July 2012
- Zhang, Dieqing; Wang, Songling; Zhu, Jian
- Applied Catalysis B: Environmental, Vol. 123-124
Constructing noble-metal-free Z-scheme photocatalytic overall water splitting systems using MoS 2 nanosheet modified CdS as a H 2 evolution photocatalyst
journal, January 2017
- Yuan, Yong-Jun; Chen, Daqin; Yang, Shuhui
- J. Mater. Chem. A, Vol. 5, Issue 40
Synthesis of one-dimensional WO 3 –Bi 2 WO 6 heterojunctions with enhanced photocatalytic activity
journal, January 2015
- Peng, Yin; Chen, Qing-Guo; Wang, Dan
- CrystEngComm, Vol. 17, Issue 3
Visible Light Photocatalytic H 2 -Production Activity of CuS/ZnS Porous Nanosheets Based on Photoinduced Interfacial Charge Transfer
journal, November 2011
- Zhang, Jun; Yu, Jiaguo; Zhang, Yimin
- Nano Letters, Vol. 11, Issue 11
Complete oxidation of acetaldehyde over a composite photocatalyst of graphitic carbon nitride and tungsten(VI) oxide under visible-light irradiation
journal, May 2014
- Jin, Zhengyuan; Murakami, Naoya; Tsubota, Toshiki
- Applied Catalysis B: Environmental, Vol. 150-151
Morphology control of one-dimensional heterojunctions for highly efficient photoanodes used for solar water splitting
journal, January 2014
- Chae, Sang Youn; Jung, Hyejin; Jeon, Hyo Sang
- Journal of Materials Chemistry A, Vol. 2, Issue 29
In situ synthesis of Bi2S3 sensitized WO3 nanoplate arrays with less interfacial defects and enhanced photoelectrochemical performance
journal, March 2016
- Liu, Canjun; Yang, Yahui; Li, Wenzhang
- Scientific Reports, Vol. 6, Issue 1
Efficient WO3 photoanodes fabricated by pulsed laser deposition for photoelectrochemical water splitting with high faradaic efficiency
journal, July 2016
- Fàbrega, C.; Murcia-López, S.; Monllor-Satoca, D.
- Applied Catalysis B: Environmental, Vol. 189
Single-Crystal Tungsten Oxide Nanosheets: Photochemical Water Oxidation in the Quantum Confinement Regime
journal, February 2012
- Waller, Mollie R.; Townsend, Troy K.; Zhao, Jing
- Chemistry of Materials, Vol. 24, Issue 4
Novel WO 3 /Sb 2 S 3 Heterojunction Photocatalyst Based on WO 3 of Different Morphologies for Enhanced Efficiency in Photoelectrochemical Water Splitting
journal, April 2016
- Zhang, Jing; Liu, Zhihua; Liu, Zhifeng
- ACS Applied Materials & Interfaces, Vol. 8, Issue 15
Yb-doped WO3 photocatalysts for water oxidation with visible light
journal, March 2014
- Liew, S. L.; Zhang, Z.; Goh, T. W. Glenn
- International Journal of Hydrogen Energy, Vol. 39, Issue 9
Constructing efficient mixed-ion perovskite solar cells based on TiO2 nanorod array
journal, January 2019
- Yang, Longkai; Wang, Xin; Mai, Xianmin
- Journal of Colloid and Interface Science, Vol. 534
Daylight Photocatalysis by Carbon-Modified Titanium Dioxide
journal, October 2003
- Sakthivel, Shanmugasundaram; Kisch, Horst
- Angewandte Chemie International Edition, Vol. 42, Issue 40, p. 4908-4911
Z-Scheme Photocatalytic Systems for Promoting Photocatalytic Performance: Recent Progress and Future Challenges
journal, April 2016
- Li, Haijin; Tu, Wenguang; Zhou, Yong
- Advanced Science, Vol. 3, Issue 11
Solvation effect promoted formation of p–n junction between WO3 and FeOOH: A high performance photoanode for water oxidation
journal, January 2016
- Huang, Jingwei; Ding, Yong; Luo, Xiao
- Journal of Catalysis, Vol. 333
WO 3 Nanorods Created by Self-Assembly of Highly Crystalline Nanowires under Hydrothermal Conditions
journal, August 2014
- Navarro, Julien R. G.; Mayence, Arnaud; Andrade, Juliana
- Langmuir, Vol. 30, Issue 34
In situ grown nickel selenide on graphene nanohybrid electrodes for high energy density asymmetric supercapacitors
journal, January 2018
- Kirubasankar, Balakrishnan; Murugadoss, Vignesh; Lin, Jing
- Nanoscale, Vol. 10, Issue 43
Microwave Popped Co(II)-Graphene Oxide Hybrid: Bifunctional Catalyst for Hydrogen Evolution Reaction and Hydrogen Storage
journal, June 2018
- Wei, Lin; Lozano, Karen; Mao, Yuanbing
- Engineered Science
Embedding Metal in the Interface of a p-n Heterojunction with a Stack Design for Superior Z-Scheme Photocatalytic Hydrogen Evolution
journal, August 2016
- Yin, Wenjie; Bai, Lijie; Zhu, Yuzhen
- ACS Applied Materials & Interfaces, Vol. 8, Issue 35
BiVO 4 /WO 3 /SnO 2 Double-Heterojunction Photoanode with Enhanced Charge Separation and Visible-Transparency for Bias-Free Solar Water-Splitting with a Perovskite Solar Cell
journal, January 2017
- Baek, Ji Hyun; Kim, Byeong Jo; Han, Gill Sang
- ACS Applied Materials & Interfaces, Vol. 9, Issue 2
A Monolithic Photovoltaic-Photoelectrochemical Device for Hydrogen Production via Water Splitting
journal, April 1998
- Khaselev, O.
- Science, Vol. 280, Issue 5362
Atomic Layer Deposition of a Submonolayer Catalyst for the Enhanced Photoelectrochemical Performance of Water Oxidation with Hematite
journal, February 2013
- Riha, Shannon C.; Klahr, Benjamin M.; Tyo, Eric C.
- ACS Nano, Vol. 7, Issue 3
WO 3 Nanoflakes for Enhanced Photoelectrochemical Conversion
journal, November 2014
- Li, Wenjie; Da, Peimei; Zhang, Yueyu
- ACS Nano, Vol. 8, Issue 11
Electron transfer reactions and flat-band potentials of tungsten(VI) oxide colloids
journal, November 1984
- Nenadovic, M. T.; Rajh, T.; Micic, O. I.
- The Journal of Physical Chemistry, Vol. 88, Issue 24
Construction of Z-scheme carbon nanodots/WO 3 with highly enhanced photocatalytic hydrogen production
journal, January 2015
- Yang, Pengju; Zhao, Jianghong; Wang, Jian
- Journal of Materials Chemistry A, Vol. 3, Issue 16
Z-schematic water splitting by the synergistic effect of a type-II heterostructure and a highly efficient oxygen evolution catalyst
journal, May 2018
- Li, Xiaoyun; Hu, Haihua; Xu, Lingbo
- Applied Surface Science, Vol. 441
Intracellular Polymer Substances Induced Conductive Polyaniline for Improved Methane Production from Anaerobic Wastewater Treatment
journal, February 2019
- Hu, Qian; Zhou, Na; Gong, Kedong
- ACS Sustainable Chemistry & Engineering, Vol. 7, Issue 6
Visible-light driven heterojunction photocatalysts for water splitting – a critical review
journal, January 2015
- Moniz, Savio J. A.; Shevlin, Stephen A.; Martin, David James
- Energy & Environmental Science, Vol. 8, Issue 3
Construction of WO 3 –g-C 3 N 4 composites as efficient photocatalysts for pharmaceutical degradation under visible light
journal, January 2017
- Zhu, Wenyu; Sun, Faqian; Goei, Ronn
- Catalysis Science & Technology, Vol. 7, Issue 12
Shape Effect and Shape Control of Polycrystalline Semiconductor Electrodes for Use in Photoelectrochemical Cells
journal, July 2010
- Choi, Kyoung-Shin
- The Journal of Physical Chemistry Letters, Vol. 1, Issue 15
Quantum dots and plasmonic Ag decorated WO3 nanorod photoanodes with enhanced photoelectrochemical performances
journal, December 2016
- Liu, Zhifeng; Wu, Jianyu; Zhang, Jing
- International Journal of Hydrogen Energy, Vol. 41, Issue 45
Extraordinary rate capability achieved by a 3D “skeleton/skin” carbon aerogel–polyaniline hybrid with vertically aligned pores
journal, January 2017
- Liu, Mingkai; Li, Bomin; Zhou, Hang
- Chemical Communications, Vol. 53, Issue 19
Mesoporous thin film WO 3 photoanode for photoelectrochemical water splitting: a sol–gel dip coating approach
journal, January 2017
- Hilliard, Samantha; Baldinozzi, Guido; Friedrich, Dennis
- Sustainable Energy & Fuels, Vol. 1, Issue 1
Electrosprayed heterojunction WO 3 /BiVO 4 films with nanotextured pillar structure for enhanced photoelectrochemical water splitting
journal, April 2015
- Mali, Mukund G.; Yoon, Hyun; Kim, Min-woo
- Applied Physics Letters, Vol. 106, Issue 15
Electrochemical Photolysis of Water at a Semiconductor Electrode
journal, July 1972
- Fujishima, Akira; Honda, Kenichi
- Nature, Vol. 238, Issue 5358, p. 37-38
WO3 and W2N nanowire arrays for photoelectrochemical hydrogen production
journal, November 2009
- Chakrapani, Vidhya; Thangala, Jyothish; Sunkara, Mahendra K.
- International Journal of Hydrogen Energy, Vol. 34, Issue 22, p. 9050-9059
Inorganic nanostructures for photoelectrochemical and photocatalytic water splitting
journal, January 2013
- Osterloh, Frank E.
- Chem. Soc. Rev., Vol. 42, Issue 6
Simultaneously Efficient Light Absorption and Charge Separation in WO 3 /BiVO 4 Core/Shell Nanowire Photoanode for Photoelectrochemical Water Oxidation
journal, January 2014
- Rao, Pratap M.; Cai, Lili; Liu, Chong
- Nano Letters, Vol. 14, Issue 2
Effect of a Cobalt-Based Oxygen Evolution Catalyst on the Stability and the Selectivity of Photo-Oxidation Reactions of a WO 3 Photoanode
journal, March 2011
- Seabold, Jason A.; Choi, Kyoung-Shin
- Chemistry of Materials, Vol. 23, Issue 5
Triple-layered nanostructured WO 3 photoanodes with enhanced photocurrent generation and superior stability for photoelectrochemical solar energy conversion
journal, January 2014
- Qi, Huan; Wolfe, Jonathan; Wang, Danping
- Nanoscale, Vol. 6, Issue 22
Monoclinic WO3 nanomultilayers with preferentially exposed (002) facets for photoelectrochemical water splitting
journal, January 2015
- Zhang, Jijie; Zhang, Peng; Wang, Tuo
- Nano Energy, Vol. 11
Vertically Aligned WO 3 Nanowire Arrays Grown Directly on Transparent Conducting Oxide Coated Glass: Synthesis and Photoelectrochemical Properties
journal, January 2011
- Su, Jinzhan; Feng, Xinjian; Sloppy, Jennifer D.
- Nano Letters, Vol. 11, Issue 1
Nitrogen Doping of Reactively Sputtered Tungsten Oxide Films
journal, January 2005
- Paluselli, Daniela; Marsen, Bjorn; Miller, Eric L.
- Electrochemical and Solid-State Letters, Vol. 8, Issue 11
Highly Efficient Photoelectrochemical Water Splitting from Hierarchical WO 3 /BiVO 4 Nanoporous Sphere Arrays
journal, November 2017
- Zhou, Yangen; Zhang, Leyuan; Lin, Linhan
- Nano Letters, Vol. 17, Issue 12
Controlled fabrication of hierarchical WO 3 hydrates with excellent adsorption performance
journal, January 2014
- Liu, Baixiong; Wang, Jinshu; Wu, Junshu
- J. Mater. Chem. A, Vol. 2, Issue 6
Mg-doped Ta 3 N 5 nanorods coated with a conformal CoOOH layer for water oxidation: bulk and surface dual modification of photoanodes
journal, January 2017
- Pei, Lang; Xu, Zhe; Shi, Zhan
- Journal of Materials Chemistry A, Vol. 5, Issue 38
Assembling graphitic-carbon-nitride with cobalt-oxide-phosphate to construct an efficient hybrid photocatalyst for water splitting application
journal, January 2013
- Lee, Rui-Lin; Tran, Phong D.; Pramana, Stevin S.
- Catalysis Science & Technology, Vol. 3, Issue 7
CdS quantum dots sensitized platelike WO3 photoelectrodes with a TiO2 buffer-layer
journal, April 2014
- Liu, Canjun; Li, Yaomin; Li, Wenzhang
- Materials Letters, Vol. 120
Morphology-Tailored Synthesis of Tungsten Trioxide (Hydrate) Thin Films and Their Photocatalytic Properties
journal, January 2011
- Jiao, Zhihui; Wang, Jinmin; Ke, Lin
- ACS Applied Materials & Interfaces, Vol. 3, Issue 2
In Situ Loading Transition Metal Oxide Clusters on TiO 2 Nanosheets As Co-catalysts for Exceptional High Photoactivity
journal, August 2013
- Liu, Lichen; Ji, Zeyang; Zou, Weixin
- ACS Catalysis, Vol. 3, Issue 9
Heterostructured WO 3 @CoWO 4 bilayer nanosheets for enhanced visible-light photo, electro and photoelectro-chemical oxidation of water
journal, January 2018
- Zhang, Huayang; Tian, Wenjie; Li, Yunguo
- Journal of Materials Chemistry A, Vol. 6, Issue 15
In Situ Growth of Metal Particles on 3D Urchin-like WO 3 Nanostructures
journal, April 2012
- Xi, Guangcheng; Ye, Jinhua; Ma, Qiang
- Journal of the American Chemical Society, Vol. 134, Issue 15
A simple one-step hydrothermal synthesis of cobalt nickel selenide/graphene nanohybrid as an advanced platinum free counter electrode for dye sensitized solar cell
journal, July 2019
- Murugadoss, Vignesh; Panneerselvam, Pratheep; Yan, Chao
- Electrochimica Acta, Vol. 312
Temperature-controlled evolution of microstructures that promote charge separation in a TaON photoanode for enhanced solar energy conversion
journal, January 2017
- Pei, Lang; Xu, Zhe; Yan, Shicheng
- Journal of Materials Chemistry A, Vol. 5, Issue 25
Photocatalytic properties of WO3 nanoparticles obtained by precipitation in presence of urea as complexing agent
journal, May 2011
- Sánchez Martínez, D.; Martínez-de la Cruz, A.; López Cuéllar, E.
- Applied Catalysis A: General, Vol. 398, Issue 1-2
Facile Preparation of Platelike Tungsten Oxide Thin Film Electrodes with High Photoelectrode Activity
journal, October 2011
- Amano, Fumiaki; Tian, Min; Wu, Guosheng
- ACS Applied Materials & Interfaces, Vol. 3, Issue 10
Nanoporous BiVO4 Photoanodes with Dual-Layer Oxygen Evolution Catalysts for Solar Water Splitting
journal, February 2014
- Kim, Tae Woo; Choi, Kyoung-Shin
- Science, Vol. 343, Issue 6174, p. 990-994
Facile preparation of Z-scheme WO 3 /g-C 3 N 4 composite photocatalyst with enhanced photocatalytic performance under visible light
journal, January 2017
- Cui, Lifeng; Ding, Xiang; Wang, Yangang
- Applied Surface Science, Vol. 391
Preparation and photoelectrocatalytic activity of a nano-structured WO3 platelet film
journal, January 2008
- Yagi, Masayuki; Maruyama, Syou; Sone, Koji
- Journal of Solid State Chemistry, Vol. 181, Issue 1
Highly efficient uranium adsorption by salicylaldoxime/polydopamine graphene oxide nanocomposites
journal, January 2018
- Qian, Yongxin; Yuan, Yihui; Wang, Heliang
- Journal of Materials Chemistry A, Vol. 6, Issue 48
Photoelectrochemical splitting of natural seawater with α-Fe 2 O 3 /WO 3 nanorod arrays
journal, February 2016
- Li, Yuangang; Feng, Juan; Li, Huajing
- International Journal of Hydrogen Energy, Vol. 41, Issue 7
Transparent Nanoparticulate FeOOH Improves the Performance of a WO 3 Photoanode in a Tandem Water-Splitting Device
journal, May 2016
- Kwong, Wai Ling; Lee, Cheng Choo; Messinger, Johannes
- The Journal of Physical Chemistry C, Vol. 120, Issue 20
Highly Active GaN-Stabilized Ta 3 N 5 Thin-Film Photoanode for Solar Water Oxidation
journal, March 2017
- Zhong, Miao; Hisatomi, Takashi; Sasaki, Yutaka
- Angewandte Chemie International Edition, Vol. 56, Issue 17
Engineering heterogeneous semiconductors for solar water splitting
journal, January 2015
- Li, Xin; Yu, Jiaguo; Low, Jingxiang
- Journal of Materials Chemistry A, Vol. 3, Issue 6
Suppressing Charge Recombination and Ultraviolet Light Degradation of Perovskite Solar Cells Using Silicon Oxide Passivation
journal, May 2019
- Ren, Jing; Luo, Qiang; Hou, Qinzhi
- ChemElectroChem, Vol. 6, Issue 12
Novel framework g-C 3 N 4 film as efficient photoanode for photoelectrochemical water splitting
journal, July 2017
- Lu, Xue; Liu, Zhifeng; Li, Junwei
- Applied Catalysis B: Environmental, Vol. 209
Role of Interfaces in Two-Dimensional Photocatalyst for Water Splitting
journal, January 2018
- Su, Tongming; Shao, Qian; Qin, Zuzeng
- ACS Catalysis, Vol. 8, Issue 3
Highly efficient photocatalyst based on all oxides WO3/Cu2O heterojunction for photoelectrochemical water splitting
journal, February 2017
- Zhang, Jing; Ma, Haipeng; Liu, Zhifeng
- Applied Catalysis B: Environmental, Vol. 201
Magnetic and electrochemical behaviour of cobalt doped tungsten oxide (WO3) nanomaterials by microwave irradiation method
journal, December 2016
- Hariharan, V.; Aroulmoji, V.; Prabakaran, K.
- Journal of Alloys and Compounds, Vol. 689
Flame synthesis of WO3 nanotubes and nanowires for efficient photoelectrochemical water-splitting
journal, January 2013
- Rao, Pratap M.; Cho, In Sun; Zheng, Xiaolin
- Proceedings of the Combustion Institute, Vol. 34, Issue 2
Two-step hydrothermally synthesized carbon nanodots/WO 3 photocatalysts with enhanced photocatalytic performance
journal, January 2017
- Song, Bo; Wang, Tingting; Sun, Honggang
- Dalton Transactions, Vol. 46, Issue 45
Solution-processed yolk–shell-shaped WO 3 /BiVO 4 heterojunction photoelectrodes for efficient solar water splitting
journal, January 2018
- Jin, Bingjun; Jung, Eunji; Ma, Ming
- Journal of Materials Chemistry A, Vol. 6, Issue 6
In situ construction of hierarchical WO3/g-C3N4 composite hollow microspheres as a Z-scheme photocatalyst for the degradation of antibiotics
journal, January 2018
- Xiao, Tingting; Tang, Zheng; Yang, Yong
- Applied Catalysis B: Environmental, Vol. 220
Photoelectrochemical Water Splitting at Semiconductor Electrodes: Fundamental Problems and New Perspectives
journal, May 2014
- Peter, Laurence M.; Upul Wijayantha, K. G.
- ChemPhysChem, Vol. 15, Issue 10
3D WO 3 /BiVO 4 /Cobalt Phosphate Composites Inverse Opal Photoanode for Efficient Photoelectrochemical Water Splitting
journal, February 2017
- Zhang, Haifeng; Zhou, Weiwei; Yang, Yaping
- Small, Vol. 13, Issue 16
Three-dimensional core-shell Fe3O4/Polyaniline coaxial heterogeneous nanonets: Preparation and high performance supercapacitor electrodes
journal, August 2019
- Ma, Yong; Hou, Chunping; Zhang, Hepeng
- Electrochimica Acta, Vol. 315
Enhanced Visible Light Activity of Single-Crystalline WO 3 Microplates for Photoelectrochemical Water Oxidation
journal, April 2016
- Park, Mira; Seo, Jong Hyeok; Song, Hyunjoon
- The Journal of Physical Chemistry C, Vol. 120, Issue 17
Highly efficient photoelectrochemical water splitting using a thin film photoanode of BiVO4/SnO2/WO3 multi-composite in a carbonate electrolyte
journal, January 2012
- Saito, Rie; Miseki, Yugo; Sayama, Kazuhiro
- Chemical Communications, Vol. 48, Issue 32
WO 3 Mesoporous Nanobelts towards Efficient Photoelectrocatalysts for Water Splitting
journal, November 2017
- Song, Kai; Gao, Fengmei; Yang, Weiyou
- ChemElectroChem, Vol. 5, Issue 2
ZnWO 4 /WO 3 Composite for Improving Photoelectrochemical Water Oxidation
journal, July 2013
- Leonard, Kevin C.; Nam, Ki Min; Lee, Heung Chan
- The Journal of Physical Chemistry C, Vol. 117, Issue 31
In situ formation of CuWO4/WO3 heterojunction plates array films with enhanced photoelectrochemical properties
journal, June 2015
- Zhan, Faqi; Li, Jie; Li, Wenzhang
- International Journal of Hydrogen Energy, Vol. 40, Issue 20
Enhancing Low-Bias Performance of Hematite Photoanodes for Solar Water Splitting by Simultaneous Reduction of Bulk, Interface, and Surface Recombination Pathways
journal, December 2015
- Cho, In Sun; Han, Hyun Soo; Logar, Manca
- Advanced Energy Materials, Vol. 6, Issue 4
A heterojunction strategy to improve the visible light sensitive water splitting performance of photocatalytic materials
journal, January 2018
- Afroz, Khurshida; Moniruddin, Md; Bakranov, Nurlan
- Journal of Materials Chemistry A, Vol. 6, Issue 44
New BiVO 4 Dual Photoanodes with Enriched Oxygen Vacancies for Efficient Solar-Driven Water Splitting
journal, March 2018
- Wang, Songcan; Chen, Peng; Bai, Yang
- Advanced Materials, Vol. 30, Issue 20
Growth of nanowire superlattice structures for nanoscale photonics and electronics
journal, February 2002
- Gudiksen, Mark S.; Lauhon, Lincoln J.; Wang, Jianfang
- Nature, Vol. 415, Issue 6872, p. 617-620
A nanostructured chromium( iii ) oxide/tungsten( vi ) oxide p–n junction photoanode toward enhanced efficiency for water oxidation
journal, January 2015
- Hu, Zhuofeng; Xu, Mingkun; Shen, Zhurui
- Journal of Materials Chemistry A, Vol. 3, Issue 26
Improved Charge Separation in WO3/CuWO4 Composite Photoanodes for Photoelectrochemical Water Oxidation
journal, May 2016
- Wang, Danping; Bassi, Prince; Qi, Huan
- Materials, Vol. 9, Issue 5
Nanostructured WO 3 /BiVO 4 Heterojunction Films for Efficient Photoelectrochemical Water Splitting
journal, May 2011
- Su, Jinzhan; Guo, Liejin; Bao, Ningzhong
- Nano Letters, Vol. 11, Issue 5
Photocatalytic Water Splitting with Suspended Calcium Niobium Oxides: Why Nanoscale is Better than Bulk – A Kinetic Analysis
journal, January 2012
- Sabio, Erwin M.; Chamousis, Rachel L.; Browning, Nigel D.
- The Journal of Physical Chemistry C, Vol. 116, Issue 4
Urchin-like NiO–NiCo 2 O 4 heterostructure microsphere catalysts for enhanced rechargeable non-aqueous Li–O 2 batteries
journal, January 2019
- Zhao, Wen; Li, Xiaomin; Yin, Rui
- Nanoscale, Vol. 11, Issue 1
Sub-nano CoO x attached onto WO 3 for efficient photocatalytic and photoelectrochemical water oxidation
journal, January 2017
- Huang, Xiubing; Zhao, Guixia; Wang, Ge
- Journal of Materials Chemistry A, Vol. 5, Issue 47
Facile growth of aligned WO3 nanorods on FTO substrate for enhanced photoanodic water oxidation activity
journal, January 2013
- Kalanur, Shankara Sharanappa; Hwang, Yun Jeong; Chae, Sang Youn
- Journal of Materials Chemistry A, Vol. 1, Issue 10
Size effects of WO3 nanocrystals for photooxidation of water in particulate suspension and photoelectrochemical film systems
journal, May 2009
- Hong, Suk Joon; Jun, Hwichan; Borse, Pramod H.
- International Journal of Hydrogen Energy, Vol. 34, Issue 8
Gold/WO3 nanocomposite photoanodes for plasmonic solar water splitting
journal, April 2016
- Hu, Dianyi; Diao, Peng; Xu, Di
- Nano Research, Vol. 9, Issue 6
Photoelectrochemical cells with tungsten trioxide/Mo-doped BiVO4 bilayers
journal, January 2012
- Zhang, Kan; Shi, Xin-Jian; Kim, Jung Kyu
- Physical Chemistry Chemical Physics, Vol. 14, Issue 31
Direct splitting of water under visible light irradiation with an oxide semiconductor photocatalyst
journal, December 2001
- Zou, Zhigang; Ye, Jinhua; Sayama, Kazuhiro
- Nature, Vol. 414, Issue 6864
Highly Efficient CdS/WO 3 Photocatalysts: Z-Scheme Photocatalytic Mechanism for Their Enhanced Photocatalytic H 2 Evolution under Visible Light
journal, September 2014
- Zhang, Li J.; Li, Shuo; Liu, Bing K.
- ACS Catalysis, Vol. 4, Issue 10
Synergistic Hematite-Fullerene Electron-Extracting Layers for Improved Efficiency and Stability in Perovskite Solar Cells
journal, January 2018
- Hou, Qinzhi; Ren, Jing; Chen, Haijun
- ChemElectroChem, Vol. 5, Issue 5
Crystallization of Tungsten Trioxide Having Small Mesopores: Highly Efficient Photoanode for Visible-Light-Driven Water Oxidation
journal, October 2013
- Chandra, Debraj; Saito, Kenji; Yui, Tatsuto
- Angewandte Chemie International Edition, Vol. 52, Issue 48
Heterogeneous interface induced formation of balsam pear-like PPy for high performance supercapacitors
journal, June 2019
- Yang, Lijia; Shi, Minjie; Jiang, Jintian
- Materials Letters, Vol. 244
A tree-like nanoporous WO 3 photoanode with enhanced charge transport efficiency for photoelectrochemical water oxidation
journal, January 2015
- Shin, Sun; Han, Hyun Soo; Kim, Ju Seong
- Journal of Materials Chemistry A, Vol. 3, Issue 24
WO3 nanoflakes decorated with CuO clusters for enhanced photoelectrochemical water splitting
journal, April 2018
- Wang, Chongwu; Tang, Jianfeng; Zhang, Xinyu
- Progress in Natural Science: Materials International, Vol. 28, Issue 2
Sol-gel synthesized hexagonal boron nitride/titania nanocomposites with enhanced photocatalytic activity
journal, January 2019
- Sheng, Yuanyuan; Yang, Jie; Wang, Fang
- Applied Surface Science, Vol. 465
Nanoscale Strontium Titanate Photocatalysts for Overall Water Splitting
journal, July 2012
- Townsend, Troy K.; Browning, Nigel D.; Osterloh, Frank E.
- ACS Nano, Vol. 6, Issue 8
Facile Fabrication of WO 3 Nanoplates Thin Films with Dominant Crystal Facet of (002) for Water Splitting
journal, October 2014
- Zheng, Jin You; Song, Guang; Hong, Jisang
- Crystal Growth & Design, Vol. 14, Issue 11
Enhancement of Photocatalytic Water Oxidation Activity on IrO x −ZnO/Zn 2− x GeO 4− x −3 y N 2 y Catalyst with the Solid Solution Phase Junction
journal, June 2010
- Ma, Baojun; Yang, Jinhui; Han, Hongxian
- The Journal of Physical Chemistry C, Vol. 114, Issue 29
Recent progress on perovskite materials in photovoltaic and water splitting applications
journal, March 2018
- Moniruddin, Md; Ilyassov, Baurzhan; Zhao, Xiao
- Materials Today Energy, Vol. 7
Highly Efficient Photocatalytic Z-Scheme Hydrogen Production over Oxygen-Deficient WO3–x Nanorods supported Zn0.3Cd0.7S Heterostructure
journal, July 2017
- Yousaf, Ammar Bin; Imran, M.; Zaidi, Syed Javaid
- Scientific Reports, Vol. 7, Issue 1
Polyborosilazane derived ceramics - Nitrogen sulfur dual doped graphene nanocomposite anode for enhanced lithium ion batteries
journal, February 2019
- Idrees, Muhammad; Batool, Saima; Kong, Jie
- Electrochimica Acta, Vol. 296
Surface intercalated spherical MoS 2x Se 2(1−x) nanocatalysts for highly efficient and durable hydrogen evolution reactions
journal, January 2019
- Lin, Bo; Lin, Zhiping; Chen, Shougang
- Dalton Transactions, Vol. 48, Issue 23
Refining waste hardmetals into tungsten oxide nanosheets via facile method
journal, April 2016
- Li, Zhifei; Zheng, Guangwei; Wang, Jinshu
- Journal of Nanoparticle Research, Vol. 18, Issue 4
An overview of lead-free piezoelectric materials and devices
journal, January 2018
- Wei, Huige; Wang, Hui; Xia, Yijie
- Journal of Materials Chemistry C, Vol. 6, Issue 46
Domain-engineered BiFeO3 thin-film photoanodes for highly enhanced ferroelectric solar water splitting
journal, June 2017
- Song, Jaesun; Kim, Taemin Ludvic; Lee, Jongmin
- Nano Research, Vol. 11, Issue 2
Artificial Photosynthetic Z-scheme Photocatalyst for Hydrogen Evolution with High Quantum Efficiency
journal, December 2016
- Guo, Hong-Li; Du, Hong; Jiang, Yi-Fan
- The Journal of Physical Chemistry C, Vol. 121, Issue 1
Anatase TiO2 single crystals with a large percentage of reactive facets
journal, May 2008
- Yang, Hua Gui; Sun, Cheng Hua; Qiao, Shi Zhang
- Nature, Vol. 453, Issue 7195
Carbon Nanomaterials in Direct Liquid Fuel Cells
journal, April 2018
- Du, Huayun; Zhao, Cindy Xinxin; Lin, Jing
- The Chemical Record, Vol. 18, Issue 9
Biological cell template synthesis of nitrogen-doped porous hollow carbon spheres/MnO2 composites for high-performance asymmetric supercapacitors
journal, February 2019
- Du, Wei; Wang, Xiaoning; Zhan, Jie
- Electrochimica Acta, Vol. 296
Hierarchically 3D assembled strontium titanate nanomaterials for water splitting application
journal, October 2017
- Moniruddin, Md; Afroz, Khurshida; Shabdan, Yerkin
- Applied Surface Science, Vol. 419
CdSe-MoS 2 : A Quantum Size-Confined Photocatalyst for Hydrogen Evolution from Water under Visible Light
journal, May 2010
- Frame, F. Andrew; Osterloh, Frank E.
- The Journal of Physical Chemistry C, Vol. 114, Issue 23
Nanostructured photoelectrodes based on WO 3 : applications to photooxidation of aqueous electrolytes
journal, January 2013
- Bignozzi, Carlo Alberto; Caramori, Stefano; Cristino, Vito
- Chem. Soc. Rev., Vol. 42, Issue 6
Crosslinked norbornene copolymer anion exchange membrane for fuel cells
journal, June 2018
- Wang, Chao; Mo, Biming; He, Zhenfeng
- Journal of Membrane Science, Vol. 556
A facile one-step strategy for in-situ fabrication of WO3-BiVO4 nanoarrays for solar-driven photoelectrochemical water splitting applications
journal, March 2017
- Iqbal, Naseer; Khan, Ibrahim; Yamani, Zain Hassan Abdallah
- Solar Energy, Vol. 144
Significant enhancement of the photoelectrochemical activity of WO3 nanoflakes by carbon quantum dots decoration
journal, August 2016
- Shi, Weina; Zhang, Xiaofan; Brillet, Jérémie
- Carbon, Vol. 105
A theoretical study on the electronic properties of in-plane CdS/ZnSe heterostructures: type-II band alignment for water splitting
journal, January 2018
- Yang, Hongchao; Li, Jinjin; Yu, Lin
- Journal of Materials Chemistry A, Vol. 6, Issue 9
An in situ transformation approach for fabrication of BiVO4/WO3 heterojunction photoanode with high photoelectrochemical activity
journal, October 2017
- Liu, Canjun; Yang, Yahui; Li, Jie
- Chemical Engineering Journal, Vol. 326
Highly efficient water splitting by a dual-absorber tandem cell
journal, November 2012
- Brillet, Jeremie; Yum, Jun-Ho; Cornuz, Maurin
- Nature Photonics, Vol. 6, Issue 12
Enhanced photopromoted electron transfer over a bilayer WO 3 n–n heterojunction prepared by RF diode sputtering
journal, January 2017
- Chiarello, Gian Luca; Bernareggi, Massimo; Pedroni, Matteo
- J. Mater. Chem. A, Vol. 5, Issue 25
Recent Progress on Visible Light Responsive Heterojunctions for Photocatalytic Applications
journal, January 2017
- Wang, Songcan; Yun, Jung-Ho; Luo, Bin
- Journal of Materials Science & Technology, Vol. 33, Issue 1
Fabrication and photoelectrochemical property of tungsten(vi) oxide films with a flake-wall structure
journal, January 2010
- Amano, Fumiaki; Li, Ding; Ohtani, Bunsho
- Chemical Communications, Vol. 46, Issue 16
Films of WO3 plate-like arrays with oxygen vacancies proportionally controlled via rapid chemical reduction
journal, January 2018
- Liu, Yang; Yang, Yahui; Liu, Qiong
- International Journal of Hydrogen Energy, Vol. 43, Issue 1
High performance MnO@C microcages with a hierarchical structure and tunable carbon shell for efficient and durable lithium storage
journal, January 2018
- Hou, Chuanxin; Tai, Zhixin; Zhao, Lanling
- Journal of Materials Chemistry A, Vol. 6, Issue 20
Ultra-long-term cycling stability of an integrated carbon–sulfur membrane with dual shuttle-inhibiting layers of graphene “nets” and a porous carbon skin
journal, January 2018
- Liu, Mingkai; Meng, Qinghua; Yang, Zhiyuan
- Chemical Communications, Vol. 54, Issue 40
Synergistic crystal facet engineering and structural control of WO3 films exhibiting unprecedented photoelectrochemical performance
journal, June 2016
- Wang, Songcan; Chen, Hongjun; Gao, Guoping
- Nano Energy, Vol. 24
Heterostructured TiO 2 /WO 3 Nanocomposites for Photocatalytic Degradation of Toluene under Visible Light
journal, January 2017
- Zhang, Li; Qin, Mingke; Yu, Wei
- Journal of The Electrochemical Society, Vol. 164, Issue 14
Water Splitting by Tungsten Oxide Prepared by Atomic Layer Deposition and Decorated with an Oxygen-Evolving Catalyst
journal, December 2010
- Liu, Rui; Lin, Yongjing; Chou, Lien-Yang
- Angewandte Chemie, Vol. 123, Issue 2
Earth-abundant cocatalysts for semiconductor-based photocatalytic water splitting
journal, January 2014
- Ran, Jingrun; Zhang, Jun; Yu, Jiaguo
- Chem. Soc. Rev., Vol. 43, Issue 22
Flake-like NiO/WO3 p-n heterojunction photocathode for photoelectrochemical water splitting
journal, May 2018
- Wu, Peidong; Liu, Zhifeng; Chen, Dong
- Applied Surface Science, Vol. 440
Preparation of vertically aligned WO3 nanoplate array films based on peroxotungstate reduction reaction and their excellent photoelectrocatalytic performance
journal, March 2017
- Zeng, Qingyi; Li, Jinhua; Bai, Jing
- Applied Catalysis B: Environmental, Vol. 202
Evaluation of Nitrogen Doping of Tungsten Oxide for Photoelectrochemical Water Splitting
journal, March 2008
- Cole, Brian; Marsen, Bjorn; Miller, Eric
- The Journal of Physical Chemistry C, Vol. 112, Issue 13
Towards highly efficient photoanodes: boosting sunlight-driven semiconductor nanomaterials for water oxidation
journal, January 2014
- Gan, Jiayong; Lu, Xihong; Tong, Yexiang
- Nanoscale, Vol. 6, Issue 13
Influence of molybdenum doping on the structural, optical and electronic properties of WO3 for improved solar water splitting
journal, January 2018
- Kalanur, Shankara S.; Seo, Hyungtak
- Journal of Colloid and Interface Science, Vol. 509
Facile Fabrication of Sandwich Structured WO 3 Nanoplate Arrays for Efficient Photoelectrochemical Water Splitting
journal, July 2016
- Feng, Xiaoyang; Chen, Yubin; Qin, Zhixiao
- ACS Applied Materials & Interfaces, Vol. 8, Issue 28
Oxygen-deficient metal oxide nanostructures for photoelectrochemical water oxidation and other applications
journal, January 2012
- Wang, Gongming; Ling, Yichuan; Li, Yat
- Nanoscale, Vol. 4, Issue 21
Cobalt-Modified Porous Single-Crystalline LaTiO 2 N for Highly Efficient Water Oxidation under Visible Light
journal, May 2012
- Zhang, Fuxiang; Yamakata, Akira; Maeda, Kazuhiko
- Journal of the American Chemical Society, Vol. 134, Issue 20
Artificial Inorganic Leafs for Efficient Photochemical Hydrogen Production Inspired by Natural Photosynthesis
journal, November 2009
- Zhou, Han; Li, Xufan; Fan, Tongxiang
- Advanced Materials, Vol. 22, Issue 9
Enhanced photoelectrocatalytic performance of nanoporous WO3 photoanode by modification of cobalt–phosphate (Co–Pi) catalyst
journal, September 2013
- Liu, Qiang; Chen, Quanpeng; Bai, Jing
- Journal of Solid State Electrochemistry, Vol. 18, Issue 1
Facile surfactant driven fabrication of transparent WO3 photoanodes for improved photoelectrochemical properties
journal, July 2016
- Kim, Jin Hyun; Lee, Byeong Jun; Wang, Ping
- Applied Catalysis A: General, Vol. 521
Z-Scheme Water Splitting Using Two Different Semiconductor Photocatalysts
journal, June 2013
- Maeda, Kazuhiko
- ACS Catalysis, Vol. 3, Issue 7
Photocatalytic Z-Scheme Water Splitting for Independent H 2 /O 2 Production via a Stepwise Operation Employing a Vanadate Redox Mediator under Visible Light
journal, April 2017
- Miseki, Yugo; Fujiyoshi, Satoshi; Gunji, Takahiro
- The Journal of Physical Chemistry C, Vol. 121, Issue 18
Review of two-dimensional materials for photocatalytic water splitting from a theoretical perspective
journal, January 2017
- Li, Yunguo; Li, Yan-Ling; Sa, Baisheng
- Catalysis Science & Technology, Vol. 7, Issue 3
Photoelectrolysis of water using semiconducting TiO2 crystals
journal, October 1975
- Nozik, A. J.
- Nature, Vol. 257, Issue 5525
Spontaneous polarization field-enhanced charge separation for an iron oxide photo-catalyst
journal, January 2017
- Johnson, J.; Bakranov, N.; Moniruddin, M.
- New Journal of Chemistry, Vol. 41, Issue 24
Photoelectrochemical activity of NiWO4/WO3 heterojunction photoanode under visible light irradiation
journal, December 2013
- Zhu, Jing; Li, Wenzhang; Li, Jie
- Electrochimica Acta, Vol. 112
Photogenerated-carrier separation along edge dislocation of WO 3 single-crystal nanoflower photoanode
journal, January 2018
- Bu, Yuyu; Ren, Jun; Zhang, Huawei
- Journal of Materials Chemistry A, Vol. 6, Issue 18
Photoelectrochemical and physical properties of WO3 films obtained by the polymeric precursor method
journal, December 2010
- Li, Wenzhang; Li, Jie; Wang, Xuan
- International Journal of Hydrogen Energy, Vol. 35, Issue 24
Nanostructured photoelectrodes for dye-sensitized solar cells
journal, February 2011
- Zhang, Qifeng; Cao, Guozhong
- Nano Today, Vol. 6, Issue 1
An Electrochemically Treated BiVO 4 Photoanode for Efficient Photoelectrochemical Water Splitting
journal, June 2017
- Wang, Songcan; Chen, Peng; Yun, Jung-Ho
- Angewandte Chemie International Edition, Vol. 56, Issue 29
Crystallization of Tungsten Trioxide Having Small Mesopores: Highly Efficient Photoanode for Visible-Light-Driven Water Oxidation
journal, October 2013
- Chandra, Debraj; Saito, Kenji; Yui, Tatsuto
- Angewandte Chemie, Vol. 125, Issue 48
Highly Active GaN-Stabilized Ta 3 N 5 Thin-Film Photoanode for Solar Water Oxidation
journal, March 2017
- Zhong, Miao; Hisatomi, Takashi; Sasaki, Yutaka
- Angewandte Chemie, Vol. 129, Issue 17
Water Splitting by Tungsten Oxide Prepared by Atomic Layer Deposition and Decorated with an Oxygen-Evolving Catalyst
journal, December 2010
- Liu, Rui; Lin, Yongjing; Chou, Lien-Yang
- Angewandte Chemie International Edition, Vol. 50, Issue 2
Dual Oxygen and Tungsten Vacancies on a WO 3 Photoanode for Enhanced Water Oxidation
journal, August 2016
- Ma, Ming; Zhang, Kan; Li, Ping
- Angewandte Chemie, Vol. 128, Issue 39
Works referencing / citing this record:
Enhancing the photocatalytic activity of Ga 2 O 3 –TiO 2 nanocomposites using sonication amplitudes for the degradation of Rhodamine B dye
journal, December 2019
- Amdeha, Enas; El‐Salamony, Radwa A.; Al‐Sabagh, Ahmed M.
- Applied Organometallic Chemistry, Vol. 34, Issue 2
Multifunctions of Polymer Nanocomposites: Environmental Remediation, Electromagnetic Interference Shielding, And Sensing Applications
journal, January 2020
- Wei, Huige; Wang, Hui; Li, Ang
- ChemNanoMat, Vol. 6, Issue 2
Advances in Template Prepared Nano‐Oxides and their Applications: Polluted Water Treatment, Energy, Sensing and Biomedical Drug Delivery
journal, January 2020
- Zhao, Junkai; Shao, Qian; Ge, Shengsong
- The Chemical Record, Vol. 20, Issue 7
Electrical and optical properties of titanium oxynitride thin films
journal, January 2020
- Mucha, Nikhil R.; Som, Jacob; Shaji, Surabhi
- Journal of Materials Science, Vol. 55, Issue 12
Confinement of Mg Nanoparticles by Bituminous Coal and Associated Synergistic Hydrogen Storage Effect
journal, January 2020
- Song, Yanwei; Zhang, Tonghuan; Zhou, Shixue
- Journal of Materials Engineering and Performance, Vol. 29, Issue 2
2D MXenes as Co-catalysts in Photocatalysis: Synthetic Methods
journal, September 2019
- Sun, Yuliang; Meng, Xing; Dall’Agnese, Yohan
- Nano-Micro Letters, Vol. 11, Issue 1
Reversibly switching water droplets wettability on hierarchical structured Cu2S mesh for efficient oil/water separation
journal, August 2019
- Xu, Shanya; Sheng, Rui; Cao, Yali
- Scientific Reports, Vol. 9, Issue 1
Selectivity of Tungsten Oxide Synthesized by Sol-Gel Method Towards Some Volatile Organic Compounds and Gaseous Materials in a Broad Range of Temperatures
journal, January 2020
- Ramanavičius, Simonas; Petrulevičienė, Milda; Juodkazytė, Jurga
- Materials, Vol. 13, Issue 3
2D/2D WO3·H2O/g-C3N4 heterostructured assemblies for enhanced photocatalytic water decontamination via strong interfacial contact
journal, December 2019
- Li, Longfei; Jiang, Daixun; Wu, Xilu
- Journal of Materials Science, Vol. 55, Issue 10
Green Synthetic Fuels: Renewable Routes for the Conversion of Non-Fossil Feedstocks into Gaseous Fuels and Their End Uses
journal, January 2020
- Rozzi, Elena; Minuto, Francesco Demetrio; Lanzini, Andrea
- Energies, Vol. 13, Issue 2
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal