Ab Initio Molecular Dynamics Reveal Spectroscopic Siblings and Ion Pairing as New Challenges for Elucidating Prenucleation Aluminum Speciation
Abstract
We report the characterization of prenucleation species is essential to understand crystallization mechanisms across many chemical systems and often involves the use of vibrational spectroscopy. Nowhere is this more evident than in the development of “green” aluminum processing technologies, where detailed understanding of the speciation of aluminum and its polynuclear analogues in highly alkaline, low water solutions is elusive. The aluminate anion Al(OH)4– predominates in alkaline conditions, yet equilibrium with dimeric species, either μ-oxo Al2O(OH)62– or di-μ-hydroxo Al2(OH)82–, can be assumed. Using ab initio molecular dynamics with full solvation and the presence of counterions, this work reconciles previous contradictory studies that had concluded only a single species under relevant solution conditions. We reveal that the two dimers are energetically separated by 2 kcal/mol in pure water but that the stability of each can be reversed by ion pairing expected in saturated salt solutions. Simulated Raman and IR spectra for each species (accounting for anharmonicity and the fluctuating solvating environment) provide the first proof that the considered species are “spectroscopic siblings”, whose multiple overlapping bands prevent definitive assertions in terms of speciation when compared to the experimental spectra. Lastly, these observations are likely to hold in higher order aluminate oligomers andmore »
- Authors:
-
- Washington State Univ., Pullman, WA (United States)
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States)
- Washington State Univ., Pullman, WA (United States); Pacific Northwest National Lab. (PNNL), Richland, WA (United States)
- Washington River Protection Solutions, LLC, Richland, WA (United States)
- Publication Date:
- Research Org.:
- Pacific Northwest National Laboratory (PNNL), Richland, WA (United States); Energy Frontier Research Centers (EFRC) (United States). Interfacial Dynamics in Radioactive Environments and Materials (IDREAM); Univ. of California, Berkeley, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); USDOE Office of Science (SC), Biological and Environmental Research (BER)
- Contributing Org.:
- IDREAM (Interfacial Dynamics in Radioactive Environments and Materials); Environmental Molecular Sciences Laboratory (EMSL)
- OSTI Identifier:
- 1504422
- Alternate Identifier(s):
- OSTI ID: 1596344
- Report Number(s):
- PNNL-SA-132755
Journal ID: ISSN 1520-6106
- Grant/Contract Number:
- AC05-76RL01830; AC02-05CH11231
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Physical Chemistry. B, Condensed Matter, Materials, Surfaces, Interfaces and Biophysical Chemistry
- Additional Journal Information:
- Journal Volume: 122; Journal Issue: 29; Journal ID: ISSN 1520-6106
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Pouvreau, Maxime, Dembowski, Mateusz, Clark, Sue B., Reynolds, Jacob G., Rosso, Kevin M., Schenter, Gregory K., Pearce, Carolyn I., and Clark, Aurora E. Ab Initio Molecular Dynamics Reveal Spectroscopic Siblings and Ion Pairing as New Challenges for Elucidating Prenucleation Aluminum Speciation. United States: N. p., 2018.
Web. doi:10.1021/acs.jpcb.8b04377.
Pouvreau, Maxime, Dembowski, Mateusz, Clark, Sue B., Reynolds, Jacob G., Rosso, Kevin M., Schenter, Gregory K., Pearce, Carolyn I., & Clark, Aurora E. Ab Initio Molecular Dynamics Reveal Spectroscopic Siblings and Ion Pairing as New Challenges for Elucidating Prenucleation Aluminum Speciation. United States. https://doi.org/10.1021/acs.jpcb.8b04377
Pouvreau, Maxime, Dembowski, Mateusz, Clark, Sue B., Reynolds, Jacob G., Rosso, Kevin M., Schenter, Gregory K., Pearce, Carolyn I., and Clark, Aurora E. Sun .
"Ab Initio Molecular Dynamics Reveal Spectroscopic Siblings and Ion Pairing as New Challenges for Elucidating Prenucleation Aluminum Speciation". United States. https://doi.org/10.1021/acs.jpcb.8b04377. https://www.osti.gov/servlets/purl/1504422.
@article{osti_1504422,
title = {Ab Initio Molecular Dynamics Reveal Spectroscopic Siblings and Ion Pairing as New Challenges for Elucidating Prenucleation Aluminum Speciation},
author = {Pouvreau, Maxime and Dembowski, Mateusz and Clark, Sue B. and Reynolds, Jacob G. and Rosso, Kevin M. and Schenter, Gregory K. and Pearce, Carolyn I. and Clark, Aurora E.},
abstractNote = {We report the characterization of prenucleation species is essential to understand crystallization mechanisms across many chemical systems and often involves the use of vibrational spectroscopy. Nowhere is this more evident than in the development of “green” aluminum processing technologies, where detailed understanding of the speciation of aluminum and its polynuclear analogues in highly alkaline, low water solutions is elusive. The aluminate anion Al(OH)4– predominates in alkaline conditions, yet equilibrium with dimeric species, either μ-oxo Al2O(OH)62– or di-μ-hydroxo Al2(OH)82–, can be assumed. Using ab initio molecular dynamics with full solvation and the presence of counterions, this work reconciles previous contradictory studies that had concluded only a single species under relevant solution conditions. We reveal that the two dimers are energetically separated by 2 kcal/mol in pure water but that the stability of each can be reversed by ion pairing expected in saturated salt solutions. Simulated Raman and IR spectra for each species (accounting for anharmonicity and the fluctuating solvating environment) provide the first proof that the considered species are “spectroscopic siblings”, whose multiple overlapping bands prevent definitive assertions in terms of speciation when compared to the experimental spectra. Lastly, these observations are likely to hold in higher order aluminate oligomers and as such present a massive challenge toward understanding the crystallization mechanisms relevant to aluminum processing.},
doi = {10.1021/acs.jpcb.8b04377},
journal = {Journal of Physical Chemistry. B, Condensed Matter, Materials, Surfaces, Interfaces and Biophysical Chemistry},
number = 29,
volume = 122,
place = {United States},
year = {Sun Jun 24 00:00:00 EDT 2018},
month = {Sun Jun 24 00:00:00 EDT 2018}
}
Web of Science
Figures / Tables:
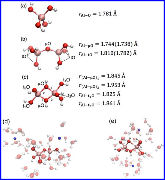 Figure 1: AIMD snapshots of (a) Al(OH)4−, (b) Al2O(OH)62−, and (c) Al2(OH)82− and average Al−O bond lengths from the simulation trajectory. (b) Al−O bond lengths in the K2[Al2O(OH)6] crystal are in parentheses. (d,e) AIMD snapshots of the ion-paired species Na+· Al2O(OH)62− (with the second, separate Na+) and 2Na+··· Al2(OH)82−.
Figure 1: AIMD snapshots of (a) Al(OH)4−, (b) Al2O(OH)62−, and (c) Al2(OH)82− and average Al−O bond lengths from the simulation trajectory. (b) Al−O bond lengths in the K2[Al2O(OH)6] crystal are in parentheses. (d,e) AIMD snapshots of the ion-paired species Na+· Al2O(OH)62− (with the second, separate Na+) and 2Na+··· Al2(OH)82−.
Works referenced in this record:
The apparent solubility of aluminum (III) in Hanford high-level waste
journal, December 2012
- Reynolds, Jacob G.
- Journal of Environmental Science and Health, Part A, Vol. 47, Issue 14
Conversion of Coarse Gibbsite Remaining in Hanford Nuclear Waste Tank Heels to Solid Sodium Aluminate [NaAl(OH) 4 ·1.5H 2 O]
journal, August 2014
- Herting, Daniel L.; Reynolds, Jacob G.; Barton, W. Blaine
- Industrial & Engineering Chemistry Research, Vol. 53, Issue 36
Gibbsite Solubility in Hanford Nuclear Waste Approached from above and below Saturation
journal, May 2016
- Reynolds, Jacob G.; McCoskey, Jacob K.; Herting, Daniel L.
- Industrial & Engineering Chemistry Research, Vol. 55, Issue 19
The structure of Al(III) in strongly alkaline aluminate solutions — A review
journal, May 2009
- Sipos, Pál
- Journal of Molecular Liquids, Vol. 146, Issue 1-2
Structure of Aqueous Sodium Aluminate Solutions: A Solution X-ray Diffraction Study
journal, October 1998
- Radnai, Tamás; May, Peter M.; Hefter, Glenn T.
- The Journal of Physical Chemistry A, Vol. 102, Issue 40
Dielectric Relaxation of Concentrated Alkaline Aluminate Solutions
journal, July 2002
- Buchner, Richard; Sipos, Pál; Hefter, Glenn
- The Journal of Physical Chemistry A, Vol. 106, Issue 28
The Raman Spectra and Structures of Aluminate and Zincate Ions
journal, March 1952
- Lippincott, Ellis R.; Psellos, James A.; Tobin, Marvin C.
- The Journal of Chemical Physics, Vol. 20, Issue 3
Structure of the aluminate ion in solutions at high pH
journal, October 1970
- Moolenaar, Robert J.; Evans, John Cyril; McKeever, L. D.
- The Journal of Physical Chemistry, Vol. 74, Issue 20
Structure and Behaviour of Aluminate Ions in Solution
journal, February 1974
- Eremin, N. I.; Volokhov, Yu A.; Mironov, V. E.
- Russian Chemical Reviews, Vol. 43, Issue 2
Spectroscopy of Concentrated Sodium Aluminate Solutions
journal, February 1998
- Watling, Helen
- Applied Spectroscopy, Vol. 52, Issue 2
Ionic structure in caustic aluminate solutions and the precipitation of gibbsite
journal, January 1998
- Watling, Helen R.; Fleming, Sean D.; van Bronswijk, Wilhelm
- Journal of the Chemical Society, Dalton Transactions, Issue 23
Raman, IR, and 27 Al-MAS-NMR Spectroscopic Studies of Sodium (Hydroxy)Aluminates
journal, April 1999
- Watling, H. R.; Sipos, P. M.; Byrne, L.
- Applied Spectroscopy, Vol. 53, Issue 4
Raman Study of Aluminum Speciation in Simulated Alkaline Nuclear Waste
journal, June 2002
- Johnston, Cliff T.; Agnew, Stephen F.; Schoonover, Jon R.
- Environmental Science & Technology, Vol. 36, Issue 11
Raman spectroscopy and multivariate curve resolution of concentrated Al2O3-Na2O-H2O solutions
journal, January 2003
- Schoonover, Jon R.; Zhang, Shuliang L.; Johnston, Cliff T.
- Journal of Raman Spectroscopy, Vol. 34, Issue 6
Quantitative determination of an aluminate dimer in concentrated alkaline aluminate solutions by Raman spectroscopy
journal, January 2006
- Sipos, Pál; May, Peter M.; Hefter, Glenn
- Dalton Trans., Issue 2
Influence of thermal history on conversion of aluminate species in sodium aluminate solution
journal, October 2014
- Li, Xiao-bin; Zhao, Dong-feng; Yang, Shuai-shuai
- Transactions of Nonferrous Metals Society of China, Vol. 24, Issue 10
Theoretical Investigation of the Nature of Aluminum-Containing Species Present in Alkaline Solution
journal, December 1998
- Gale, Julian D.; Rohl, Andrew L.; Watling, Helen R.
- The Journal of Physical Chemistry B, Vol. 102, Issue 50
An investigation of the mechanism of gibbsite nucleation using molecular modelling
journal, May 1996
- Gerson, Andrea R.; Ralston, John; Smart, Roger St. C.
- Colloids and Surfaces A: Physicochemical and Engineering Aspects, Vol. 110, Issue 1
Investigation on the decomposition process of sodium aluminate solution by spectroscopic and theoretical calculation
journal, July 2018
- Liu, Wei; Huang, Ya-ling; Yin, Zhou-lan
- Journal of Molecular Liquids, Vol. 261
Growth Mechanisms and Kinetics of Gibbsite Crystallization: Experimental and Quantum Chemical Study
journal, May 2012
- Li, Jun; Addai-Mensah, Jonas; Thilagam, Alagu
- Crystal Growth & Design, Vol. 12, Issue 6
DFT studies of Al–O Raman vibrational frequencies for aquated aluminium species
journal, October 2010
- Lu, Bang-Mei; Jin, Xiao-Yan; Tang, Jie
- Journal of Molecular Structure, Vol. 982, Issue 1-3
Theoretical studies on aluminate and sodium aluminate species in models for aqueous solution; Al(OH) 3 , Al(OH) (super -) 4 , and NaAl(OH) 4
journal, October 1999
- Tossell, J. A.
- American Mineralogist, Vol. 84, Issue 10
The Crystal Structure of the Potassium Aluminate K2[Al2O(OH)6].
journal, January 1966
- Johansson, Georg; Hope, Håkon; Nevald, R.
- Acta Chemica Scandinavica, Vol. 20
Molecular Models of Hydroxide, Oxyhydroxide, and Clay Phases and the Development of a General Force Field
journal, January 2004
- Cygan, Randall T.; Liang, Jian-Jie; Kalinichev, Andrey G.
- The Journal of Physical Chemistry B, Vol. 108, Issue 4
Computer simulations of NaCl association in polarizable water
journal, March 1994
- Smith, David E.; Dang, Liem X.
- The Journal of Chemical Physics, Vol. 100, Issue 5
A molecular dynamics simulation of a water model with intramolecular degrees of freedom
journal, January 1987
- Teleman, Olle; Jönsson, Bo; Engström, Sven
- Molecular Physics, Vol. 60, Issue 1
Quickstep: Fast and accurate density functional calculations using a mixed Gaussian and plane waves approach
journal, April 2005
- VandeVondele, Joost; Krack, Matthias; Mohamed, Fawzi
- Computer Physics Communications, Vol. 167, Issue 2
cp2k: atomistic simulations of condensed matter systems
journal, June 2013
- Hutter, Jürg; Iannuzzi, Marcella; Schiffmann, Florian
- Wiley Interdisciplinary Reviews: Computational Molecular Science, Vol. 4, Issue 1
A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu
journal, April 2010
- Grimme, Stefan; Antony, Jens; Ehrlich, Stephan
- The Journal of Chemical Physics, Vol. 132, Issue 15
Dispersion corrected RPBE studies of liquid water
journal, August 2014
- Forster-Tonigold, Katrin; Groß, Axel
- The Journal of Chemical Physics, Vol. 141, Issue 6
Perspective: How good is DFT for water?
journal, April 2016
- Gillan, Michael J.; Alfè, Dario; Michaelides, Angelos
- The Journal of Chemical Physics, Vol. 144, Issue 13
A hybrid Gaussian and plane wave density functional scheme
journal, October 1997
- Lippert, Gerald; Hutter, JuRG; Parrinello, Michele
- Molecular Physics, Vol. 92, Issue 3
Gaussian basis sets for accurate calculations on molecular systems in gas and condensed phases
journal, September 2007
- VandeVondele, Joost; Hutter, Jürg
- The Journal of Chemical Physics, Vol. 127, Issue 11
Design of Density Functionals by Combining the Method of Constraint Satisfaction with Parametrization for Thermochemistry, Thermochemical Kinetics, and Noncovalent Interactions
journal, January 2006
- Zhao, Yan; Schultz, Nathan E.; Truhlar, Donald G.
- Journal of Chemical Theory and Computation, Vol. 2, Issue 2
Thirty years of density functional theory in computational chemistry: an overview and extensive assessment of 200 density functionals
journal, April 2017
- Mardirossian, Narbe; Head-Gordon, Martin
- Molecular Physics, Vol. 115, Issue 19
TRAVIS - A Free Analyzer and Visualizer for Monte Carlo and Molecular Dynamics Trajectories
journal, July 2011
- Brehm, Martin; Kirchner, Barbara
- Journal of Chemical Information and Modeling, Vol. 51, Issue 8
A study of the vibrational motions of water in an aqueous CaCl2 solution
journal, July 1986
- Bopp, P.
- Chemical Physics, Vol. 106, Issue 2
Vibrational analysis of glycine radical: a comparative ab initio static and dynamic study
journal, January 2009
- Carbonniere, Philippe; Dargelos, Alain; Ciofini, Ilaria
- Physical Chemistry Chemical Physics, Vol. 11, Issue 21
Works referencing / citing this record:
PageRank as a collective variable to study complex chemical transformations and their energy landscapes
journal, April 2019
- Zhou, Tiecheng; Martinez-Baez, Ernesto; Schenter, Gregory
- The Journal of Chemical Physics, Vol. 150, Issue 13
Inference of principal species in caustic aluminate solutions through solid-state spectroscopic characterization
journal, January 2020
- Dembowski, Mateusz; Prange, Micah P.; Pouvreau, Maxime
- Dalton Transactions, Vol. 49, Issue 18
Ion–ion interactions enhance aluminum solubility in alkaline suspensions of nano-gibbsite (α-Al(OH) 3 ) with sodium nitrite/nitrate
journal, January 2020
- Dembowski, Mateusz; Snyder, Michelle M.; Delegard, Calvin H.
- Physical Chemistry Chemical Physics, Vol. 22, Issue 8
$^{27}\text{Al }$ NMR chemical shift of $\text{Al}(\text{OH})_{4}^{-}$ from first principles. Assessment of error cancellation in NMR chemical shift computations in chemically distinct reference and targeted systems
text, January 2020
- Martinez-Baez, Ernesto; Feng, Rulin; Pearce, Carolyn I.
- arXiv
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal