Establishing Cost-Effective Computational Models for the Prediction of Lanthanoid Binding in [Ln(NO 3 )] 2+ (with Ln = La to Lu)
Abstract
Evaluating the efficiency of predictive methods is critical to the processes of upscaling laboratory processes to full-scale operations on an industrial scale. With regard to separation of lanthanoids, there is a considerable motivation to optimize these processes because of immediate use in nuclear fuel cycle operations, nuclear forensics applications, and rare-earth metal recovery. Efficient predictive capabilities in Gibbs free energies of reaction are essential to optimize separations and ligand design for selective binding needed for various radiochemical applications such as nuclear fuel disposition and recycling of lanthanoid fission products into useful radioisotope products. Ligand design is essential for selective binding of lanthanoids, as separating contiguous lanthanoids is challenging because of the similar behavior these elements exhibit. Modeling including electronic structure calculations of lanthanoid-containing compounds is particularly challenging because of the associated computational cost encountered with the number of electrons correlated in these systems and relativistic considerations. This study evaluates the predictive capabilities of various ab initio methods in the calculation of Gibbs free energies of reaction for [Ln(NO3)]2+ compounds (with Ln = La to Lu), as nitrates are critical in traditional separation processes utilizing nitric acid. The composite methodologies evaluated predict Gibbs free energies of reaction for [Ln(NO3)]2+ compounds withinmore »
- Authors:
-
- Research Information Technology Services, University of North Texas, 225 S. Avenue B, Denton, Texas 76201, United States, Institute for Nuclear Security, University of Tennessee, 1640 Cumberland Avenue, Knoxville, Tennessee 37996, United States
- Institute for Nuclear Security, University of Tennessee, 1640 Cumberland Avenue, Knoxville, Tennessee 37996, United States
- Department of Nuclear Engineering, University of Tennessee, 301 Middle Dr., Pasqua Nuclear Engineering Bldg., Knoxville, Tennessee 37996, United States
- Institute for Nuclear Security, University of Tennessee, 1640 Cumberland Avenue, Knoxville, Tennessee 37996, United States, Department of Nuclear Engineering, University of Tennessee, 301 Middle Dr., Pasqua Nuclear Engineering Bldg., Knoxville, Tennessee 37996, United States, Radiochemistry Center of Excellence (RCOE), University of Tennessee, 1508 Middle Dr., Ferris Hall, Knoxville, Tennessee 37996, United States, Y-12 National Security Complex, Oak Ridge, Tennessee 37830, United States
- Publication Date:
- Research Org.:
- Univ. of Tennessee, Knoxville, TN (United States)
- Sponsoring Org.:
- USDOE National Nuclear Security Administration (NNSA); USDOE Office of Science (SC); National Science Foundation (NSF)
- OSTI Identifier:
- 1491051
- Alternate Identifier(s):
- OSTI ID: 1508790
- Grant/Contract Number:
- NA0001983; AC02-05CH11231; ACI-1548562
- Resource Type:
- Published Article
- Journal Name:
- ACS Omega
- Additional Journal Information:
- Journal Name: ACS Omega Journal Volume: 4 Journal Issue: 1; Journal ID: ISSN 2470-1343
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 36 MATERIALS SCIENCE; 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; 11 NUCLEAR FUEL CYCLE AND FUEL MATERIALS; free energy; materials science; organic compounds and functional groups; quantum mechanical methods; rare earth salts; theory
Citation Formats
Peterson, Charles C., Penchoff, Deborah A., Auxier, II, John D., and Hall, Howard L. Establishing Cost-Effective Computational Models for the Prediction of Lanthanoid Binding in [Ln(NO 3 )] 2+ (with Ln = La to Lu). United States: N. p., 2019.
Web. doi:10.1021/acsomega.8b02403.
Peterson, Charles C., Penchoff, Deborah A., Auxier, II, John D., & Hall, Howard L. Establishing Cost-Effective Computational Models for the Prediction of Lanthanoid Binding in [Ln(NO 3 )] 2+ (with Ln = La to Lu). United States. https://doi.org/10.1021/acsomega.8b02403
Peterson, Charles C., Penchoff, Deborah A., Auxier, II, John D., and Hall, Howard L. Wed .
"Establishing Cost-Effective Computational Models for the Prediction of Lanthanoid Binding in [Ln(NO 3 )] 2+ (with Ln = La to Lu)". United States. https://doi.org/10.1021/acsomega.8b02403.
@article{osti_1491051,
title = {Establishing Cost-Effective Computational Models for the Prediction of Lanthanoid Binding in [Ln(NO 3 )] 2+ (with Ln = La to Lu)},
author = {Peterson, Charles C. and Penchoff, Deborah A. and Auxier, II, John D. and Hall, Howard L.},
abstractNote = {Evaluating the efficiency of predictive methods is critical to the processes of upscaling laboratory processes to full-scale operations on an industrial scale. With regard to separation of lanthanoids, there is a considerable motivation to optimize these processes because of immediate use in nuclear fuel cycle operations, nuclear forensics applications, and rare-earth metal recovery. Efficient predictive capabilities in Gibbs free energies of reaction are essential to optimize separations and ligand design for selective binding needed for various radiochemical applications such as nuclear fuel disposition and recycling of lanthanoid fission products into useful radioisotope products. Ligand design is essential for selective binding of lanthanoids, as separating contiguous lanthanoids is challenging because of the similar behavior these elements exhibit. Modeling including electronic structure calculations of lanthanoid-containing compounds is particularly challenging because of the associated computational cost encountered with the number of electrons correlated in these systems and relativistic considerations. This study evaluates the predictive capabilities of various ab initio methods in the calculation of Gibbs free energies of reaction for [Ln(NO3)]2+ compounds (with Ln = La to Lu), as nitrates are critical in traditional separation processes utilizing nitric acid. The composite methodologies evaluated predict Gibbs free energies of reaction for [Ln(NO3)]2+ compounds within 5 kcal mol–1 in most cases from the target method [CCSD(T)-FSII/cc-pwCV∞Z-DK3+SO] at a fraction of the computational cost.},
doi = {10.1021/acsomega.8b02403},
journal = {ACS Omega},
number = 1,
volume = 4,
place = {United States},
year = {Wed Jan 16 00:00:00 EST 2019},
month = {Wed Jan 16 00:00:00 EST 2019}
}
https://doi.org/10.1021/acsomega.8b02403
Web of Science
Figures / Tables:
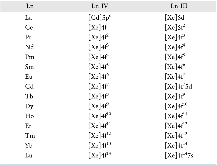 Table 1: Electronic Configuration for Ln IV and Ln III43
Table 1: Electronic Configuration for Ln IV and Ln III43
Works referenced in this record:
The role of databases in support of computational chemistry calculations
journal, October 1996
- Feller, David
- Journal of Computational Chemistry, Vol. 17, Issue 13
Review Article: The Effects of Radiation Chemistry on Solvent Extraction: 2. A Review of Fission‐Product Extraction
journal, May 2009
- Mincher, Bruce J.; Modolo, Giuseppe; Mezyk, Stephen P.
- Solvent Extraction and Ion Exchange, Vol. 27, Issue 3
Density‐functional thermochemistry. III. The role of exact exchange
journal, April 1993
- Becke, Axel D.
- The Journal of Chemical Physics, Vol. 98, Issue 7, p. 5648-5652
The synthesis and spectroscopic characterization of an aromatic uranium amidoxime complex
journal, September 2014
- Bernstein, Karl J.; Do-Thanh, Chi-Linh; Penchoff, Deborah A.
- Inorganica Chimica Acta, Vol. 421
Relativistic double-zeta, triple-zeta, and quadruple-zeta basis sets for the lanthanides La–Lu
journal, January 2010
- Gomes, André S. P.; Dyall, Kenneth G.; Visscher, Lucas
- Theoretical Chemistry Accounts, Vol. 127, Issue 4
Molpro: a general-purpose quantum chemistry program package: Molpro
journal, July 2011
- Werner, Hans-Joachim; Knowles, Peter J.; Knizia, Gerald
- Wiley Interdisciplinary Reviews: Computational Molecular Science, Vol. 2, Issue 2
Ab initio total atomization energies of small molecules — towards the basis set limit
journal, September 1996
- Martin, Jan M. L.
- Chemical Physics Letters, Vol. 259, Issue 5-6
Relativistic Douglas-Kroll-Hess theory: Relativistic DKH theory
journal, June 2011
- Reiher, Markus
- Wiley Interdisciplinary Reviews: Computational Molecular Science, Vol. 2, Issue 1
Synthesis and Molecular Structure of a Plutonium(IV) Coordination Complex: [Pu(NO 3 ) 2 {2,6-[(C 6 H 5 ) 2 P(O)CH 2 ] 2 C 5 H 3 NO} 2 ](NO 3 ) 2 •1.5H 2 O•0.5MeOH
journal, September 2000
- Bond, Evelyn M.; Duesler, Eileen N.; Paine, Robert T.
- Inorganic Chemistry, Vol. 39, Issue 18
Perspective: Fifty years of density-functional theory in chemical physics
journal, May 2014
- Becke, Axel D.
- The Journal of Chemical Physics, Vol. 140, Issue 18
Characterization of Lanthanide Complexes with Bis-1,2,3-triazole-bipyridine Ligands Involved in Actinide/Lanthanide Separation
journal, October 2016
- Muller, Julie M.; Galley, Shane S.; Albrecht-Schmitt, Thomas E.
- Inorganic Chemistry, Vol. 55, Issue 21
Basis Set Exchange: A Community Database for Computational Sciences
journal, March 2007
- Schuchardt, Karen L.; Didier, Brett T.; Elsethagen, Todd
- Journal of Chemical Information and Modeling, Vol. 47, Issue 3
Cerium(IV), Neptunium(IV), and Plutonium(IV) 1,2-Phenylenediphosphonates: Correlations and Differences between Early Transuranium Elements and Their Proposed Surrogates
journal, November 2010
- Diwu, Juan; Wang, Shuao; Liao, Zuolei
- Inorganic Chemistry, Vol. 49, Issue 21
Self‐consistent molecular orbital methods. XX. A basis set for correlated wave functions
journal, January 1980
- Krishnan, R.; Binkley, J. S.; Seeger, R.
- The Journal of Chemical Physics, Vol. 72, Issue 1
The correlation consistent composite approach (cc CA ): An alternative to the Gaussian-n methods
journal, March 2006
- DeYonker, Nathan J.; Cundari, Thomas R.; Wilson, Angela K.
- The Journal of Chemical Physics, Vol. 124, Issue 11
Comment on: “Estimating the Hartree–Fock limit from finite basis set calculations” [Jensen F (2005) Theor Chem Acc 113:267]
journal, December 2005
- Karton, Amir; Martin, Jan M. L.
- Theoretical Chemistry Accounts, Vol. 115, Issue 4
Parallel Douglas–Kroll energy and gradients in NWChem: Estimating scalar relativistic effects using Douglas–Kroll contracted basis sets
journal, January 2001
- de Jong, W. A.; Harrison, R. J.; Dixon, D. A.
- The Journal of Chemical Physics, Vol. 114, Issue 1
Synthesis, Lanthanide Coordination Chemistry, and Liquid–Liquid Extraction Performance of CMPO-Decorated Pyridine and Pyridine N -Oxide Platforms
journal, March 2013
- Rosario-Amorin, Daniel; Ouizem, Sabrina; Dickie, Diane A.
- Inorganic Chemistry, Vol. 52, Issue 6
Structural Analysis of the Complexation of Uranyl, Neptunyl, Plutonyl, and Americyl with Cyclic Imide Dioximes
journal, October 2018
- Penchoff, Deborah A.; Peterson, Charles C.; Camden, Jon P.
- ACS Omega, Vol. 3, Issue 10
Toward Chemical Accuracy in ab Initio Thermochemistry and Spectroscopy of Lanthanide Compounds: Assessing Core–Valence Correlation, Second-Order Spin–Orbit Coupling, and Higher Order Effects in Lanthanide Diatomics
journal, October 2017
- Solomonik, Victor G.; Smirnov, Alexander N.
- Journal of Chemical Theory and Computation, Vol. 13, Issue 11
NWChem: A comprehensive and scalable open-source solution for large scale molecular simulations
journal, September 2010
- Valiev, M.; Bylaska, E. J.; Govind, N.
- Computer Physics Communications, Vol. 181, Issue 9, p. 1477-1489
Structural Characteristics, Population Analysis, and Binding Energies of [An(NO 3 )] 2+ (with An = Ac to Lr)
journal, October 2018
- Penchoff, Deborah A.; Peterson, Charles C.; Quint, Mark S.
- ACS Omega, Vol. 3, Issue 10
Lanthanide Speciation in Potential SANEX and GANEX Actinide/Lanthanide Separations Using Tetra-N-Donor Extractants
journal, June 2012
- Whittaker, Daniel M.; Griffiths, Tamara L.; Helliwell, Madeleine
- Inorganic Chemistry, Vol. 52, Issue 7
Chelating properties of 2-((diphenylphosphino)methyl)pyridine N,P-dioxide and 2,6-bis((diphenylphosphino)methyl)pyridine N,P,P'-trioxide toward f-element ions
journal, May 1993
- Rapko, B. M.; Duesler, E. N.; Smith, P. H.
- Inorganic Chemistry, Vol. 32, Issue 10
Separation of Americium from Lanthanides by Purified Cyanex 301 Countercurrent Extraction in Miniature Centrifugal Contactors
journal, January 2012
- Chen, Jing; Wang, Shuwei; Xu, Chao
- Procedia Chemistry, Vol. 7
Development of Highly Selective Ligands for Separations of Actinides from Lanthanides in the Nuclear Fuel Cycle
journal, October 2011
- Lewis, Frank; Hudson, Michael; Harwood, Laurence
- Synlett, Vol. 2011, Issue 18
Ab initio approaches for the determination of heavy element energetics: Ionization energies of trivalent lanthanides (Ln = La-Eu)
journal, November 2015
- Peterson, Charles; Penchoff, Deborah A.; Wilson, Angela K.
- The Journal of Chemical Physics, Vol. 143, Issue 19
Correlation consistent basis sets for lanthanides: The atoms La–Lu
journal, August 2016
- Lu, Qing; Peterson, Kirk A.
- The Journal of Chemical Physics, Vol. 145, Issue 5
Crystallographic and Spectroscopic Characterization of Americium Complexes Containing the Bis[(phosphino)methyl]pyridine-1-oxide (NOPOPO) Ligand Platform
journal, February 2018
- Corbey, Jordan F.; Rapko, Brian M.; Wang, Zheming
- Inorganic Chemistry, Vol. 57, Issue 4

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal