Rational Construction of Compact de Novo- Designed Biliverdin-Binding Proteins
Abstract
We report the rational construction of de novo-designed biliverdin-binding proteins by first principles of protein design, informed by energy minimization modeling in Rosetta. The self-assembling tetrahelical bundles bind biliverdin IXa (BV) cofactor autocatalytically in vitro, like photosensory proteins that bind BV (and related bilins or linear tetrapyrroles) despite lacking sequence and structural homology to the natural counterparts. Upon identification of a suitable site for ligation of the cofactor to the protein scaffold, stepwise placement of residues stabilized BV within the hydrophobic core. Rosetta modeling was used in the absence of a high-resolution structure to inform the structure-function relationships of the cofactor binding pocket. Holoprotein formation stabilized BV, resulting in increased far-red BV fluorescence. Via removal of segments extraneous to cofactor stabilization or bundle stability, the initial 15 kDa de novo-designed fluorescence-activating protein was truncated without any change to its optical properties, down to a miniature 10 kDa “mini”, in which the protein scaffold extends only a half-heptad repeat beyond the hypothetical position of the bilin D-ring. This work demonstrates how highly compact holoprotein fluorochromes can be rationally constructed using de novo protein design technology and natural cofactors.
- Authors:
- Publication Date:
- Research Org.:
- Energy Frontier Research Centers (EFRC) (United States). Photosynthetic Antenna Research Center (PARC); Univ. of Pennsylvania, Philadelphia, PA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); National Inst. of Health (NIH) (United States); National Science Foundation (NSF)
- OSTI Identifier:
- 1485333
- Alternate Identifier(s):
- OSTI ID: 1508787
- Grant/Contract Number:
- SC0001035; 1R21DA040434; 1R21EY027562; 1R01NS101106; CBET 126497; MCB 1652003
- Resource Type:
- Published Article
- Journal Name:
- Biochemistry
- Additional Journal Information:
- Journal Name: Biochemistry Journal Volume: 57 Journal Issue: 49; Journal ID: ISSN 0006-2960
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 59 BASIC BIOLOGICAL SCIENCES
Citation Formats
Sheehan, Molly M., Magaraci, Michael S., Kuznetsov, Ivan A., Mancini, Joshua A., Kodali, Goutham, Moser, Christopher C., Dutton, P. Leslie, and Chow, Brian Y. Rational Construction of Compact de Novo- Designed Biliverdin-Binding Proteins. United States: N. p., 2018.
Web. doi:10.1021/acs.biochem.8b01076.
Sheehan, Molly M., Magaraci, Michael S., Kuznetsov, Ivan A., Mancini, Joshua A., Kodali, Goutham, Moser, Christopher C., Dutton, P. Leslie, & Chow, Brian Y. Rational Construction of Compact de Novo- Designed Biliverdin-Binding Proteins. United States. https://doi.org/10.1021/acs.biochem.8b01076
Sheehan, Molly M., Magaraci, Michael S., Kuznetsov, Ivan A., Mancini, Joshua A., Kodali, Goutham, Moser, Christopher C., Dutton, P. Leslie, and Chow, Brian Y. Fri .
"Rational Construction of Compact de Novo- Designed Biliverdin-Binding Proteins". United States. https://doi.org/10.1021/acs.biochem.8b01076.
@article{osti_1485333,
title = {Rational Construction of Compact de Novo- Designed Biliverdin-Binding Proteins},
author = {Sheehan, Molly M. and Magaraci, Michael S. and Kuznetsov, Ivan A. and Mancini, Joshua A. and Kodali, Goutham and Moser, Christopher C. and Dutton, P. Leslie and Chow, Brian Y.},
abstractNote = {We report the rational construction of de novo-designed biliverdin-binding proteins by first principles of protein design, informed by energy minimization modeling in Rosetta. The self-assembling tetrahelical bundles bind biliverdin IXa (BV) cofactor autocatalytically in vitro, like photosensory proteins that bind BV (and related bilins or linear tetrapyrroles) despite lacking sequence and structural homology to the natural counterparts. Upon identification of a suitable site for ligation of the cofactor to the protein scaffold, stepwise placement of residues stabilized BV within the hydrophobic core. Rosetta modeling was used in the absence of a high-resolution structure to inform the structure-function relationships of the cofactor binding pocket. Holoprotein formation stabilized BV, resulting in increased far-red BV fluorescence. Via removal of segments extraneous to cofactor stabilization or bundle stability, the initial 15 kDa de novo-designed fluorescence-activating protein was truncated without any change to its optical properties, down to a miniature 10 kDa “mini”, in which the protein scaffold extends only a half-heptad repeat beyond the hypothetical position of the bilin D-ring. This work demonstrates how highly compact holoprotein fluorochromes can be rationally constructed using de novo protein design technology and natural cofactors.},
doi = {10.1021/acs.biochem.8b01076},
journal = {Biochemistry},
number = 49,
volume = 57,
place = {United States},
year = {Fri Nov 23 00:00:00 EST 2018},
month = {Fri Nov 23 00:00:00 EST 2018}
}
https://doi.org/10.1021/acs.biochem.8b01076
Web of Science
Figures / Tables:
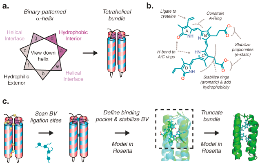 Figure 1: Engineering de novo-designed proteins to stabilize biliverdin. (a) Self-assembling single-chain tetrahelical bundles created by binary patterning of hydrophobic and hydrophobic residues with a high α-helix formation propensity, described by the helical wheel. (b) Strategy for stabilizing biliverdin within the core. (c) Holoprotein stepwise construction.
Figure 1: Engineering de novo-designed proteins to stabilize biliverdin. (a) Self-assembling single-chain tetrahelical bundles created by binary patterning of hydrophobic and hydrophobic residues with a high α-helix formation propensity, described by the helical wheel. (b) Strategy for stabilizing biliverdin within the core. (c) Holoprotein stepwise construction.
Works referenced in this record:
Phycofluor probes
journal, October 1984
- Glazer, Alexander N.; Stryer, Lubert
- Trends in Biochemical Sciences, Vol. 9, Issue 10
Origins of Fluorescence in Evolved Bacteriophytochromes
journal, September 2014
- Bhattacharya, Shyamosree; Auldridge, Michele E.; Lehtivuori, Heli
- Journal of Biological Chemistry, Vol. 289, Issue 46
Characterization of a helical protein designed from first principles
journal, August 1988
- Regan, L.; DeGrado, W. F.
- Science, Vol. 241, Issue 4868, p. 976-978
Minimal domain of bacterial phytochrome required for chromophore binding and fluorescence
journal, December 2015
- Rumyantsev, Konstantin A.; Shcherbakova, Daria M.; Zakharova, Natalia I.
- Scientific Reports, Vol. 5, Issue 1
A Bilirubin-Inducible Fluorescent Protein from Eel Muscle
journal, June 2013
- Kumagai, Akiko; Ando, Ryoko; Miyatake, Hideyuki
- Cell, Vol. 153, Issue 7
Design and synthesis of multi-haem proteins
journal, March 1994
- Robertson, Dan E.; Farid, Ramy S.; Moser, Christopher C.
- Nature, Vol. 368, Issue 6470, p. 425-432
The phytofluors: a new class of fluorescent protein probes
journal, November 1997
- Murphy, John T.; Lagarias, J.Clark
- Current Biology, Vol. 7, Issue 11, p. 870-876
The coming of age of de novo protein design
journal, September 2016
- Huang, Po-Ssu; Boyken, Scott E.; Baker, David
- Nature, Vol. 537, Issue 7620
Near-infrared fluorescent proteins engineered from bacterial phytochromes
journal, August 2015
- Shcherbakova, Daria M.; Baloban, Mikhail; Verkhusha, Vladislav V.
- Current Opinion in Chemical Biology, Vol. 27
Protein Design: The Choice of de Novo Sequences
journal, January 1997
- Beasley, James R.; Hecht, Michael H.
- Journal of Biological Chemistry, Vol. 272, Issue 4
Design, synthesis, and characterization of a photoactivatable flavocytochrome molecular maquette
journal, September 1998
- Sharp, R. E.; Moser, C. C.; Rabanal, F.
- Proceedings of the National Academy of Sciences, Vol. 95, Issue 18
De Novo Protein Design: Fully Automated Sequence Selection
journal, October 1997
- Dahiyat, Bassil I.; Mayo, Stephen L.
- Science, Vol. 278, Issue 5335
High thermodynamic stability of parametrically designed helical bundles
journal, October 2014
- Huang, Po-Ssu; Oberdorfer, Gustav; Xu, Chunfu
- Science, Vol. 346, Issue 6208
A thermodynamic scale for the helix-forming tendencies of the commonly occurring amino acids
journal, November 1990
- O'Neil, K.; DeGrado, W.
- Science, Vol. 250, Issue 4981
De novo synthetic biliprotein design, assembly and excitation energy transfer
journal, April 2018
- Mancini, Joshua A.; Sheehan, Molly; Kodali, Goutham
- Journal of The Royal Society Interface, Vol. 15, Issue 141
Biliverdin Amides Reveal Roles for Propionate Side Chains in Bilin Reductase Recognition and in Holophytochrome Assembly and Photoconversion
journal, July 2010
- Shang, Lixia; Rockwell, Nathan C.; Martin, Shelley S.
- Biochemistry, Vol. 49, Issue 29
Continuous Fluorescence Assay of Phytochrome Assembly in Vitro
journal, June 1995
- Li, Liming; Murphy, John T.; Lagarias, J. Clark
- Biochemistry, Vol. 34, Issue 24
De novo design of a hyperstable non-natural protein–ligand complex with sub-Å accuracy
journal, August 2017
- Polizzi, Nicholas F.; Wu, Yibing; Lemmin, Thomas
- Nature Chemistry, Vol. 9, Issue 12
Removal of Chromophore-Proximal Polar Atoms Decreases Water Content and Increases Fluorescence in a Near Infrared Phytofluor
journal, November 2015
- Lehtivuori, Heli; Bhattacharya, Shyamosree; Angenent-Mari, Nicolaas M.
- Frontiers in Molecular Biosciences, Vol. 2
The HP-1 maquette: From an apoprotein structure to a structured hemoprotein designed to promote redox-coupled proton exchange
journal, March 2004
- Huang, S. S.; Koder, R. L.; Lewis, M.
- Proceedings of the National Academy of Sciences, Vol. 101, Issue 15
Structural and Biochemical Characterization of the Bilin Lyase CpcS from Thermosynechococcus elongatus
journal, November 2013
- Kronfel, Christina M.; Kuzin, Alexandre P.; Forouhar, Farhad
- Biochemistry, Vol. 52, Issue 48
ROSETTALIGAND: Protein-small molecule docking with full side-chain flexibility
journal, November 2006
- Meiler, Jens; Baker, David
- Proteins: Structure, Function, and Bioinformatics, Vol. 65, Issue 3
Defining the Bilin Lyase Domain: Lessons from the Extended Phytochrome Superfamily †
journal, November 2000
- Wu, Shu-Hsing; Lagarias, J. Clark
- Biochemistry, Vol. 39, Issue 44
Blue protein with red fluorescence
journal, September 2016
- Ghosh, Swagatha; Yu, Chi-Li; Ferraro, Daniel J.
- Proceedings of the National Academy of Sciences, Vol. 113, Issue 41
Mammalian Expression of Infrared Fluorescent Proteins Engineered from a Bacterial Phytochrome
journal, May 2009
- Shu, X.; Royant, A.; Lin, M. Z.
- Science, Vol. 324, Issue 5928
De novo design of a fluorescence-activating β-barrel
journal, September 2018
- Dou, Jiayi; Vorobieva, Anastassia A.; Sheffler, William
- Nature, Vol. 561, Issue 7724
The Biliverdin Chromophore Binds Covalently to a Conserved Cysteine Residue in the N-Terminus of Agrobacterium Phytochrome Agp1 †
journal, March 2004
- Lamparter, Tilman; Carrascal, Montserrat; Michael, Norbert
- Biochemistry, Vol. 43, Issue 12
Computational de novo design of a four-helix bundle protein-DND_4HB: De Novo Design of a Helical Bundle
journal, November 2014
- Murphy, Grant S.; Sathyamoorthy, Bharatwaj; Der, Bryan S.
- Protein Science, Vol. 24, Issue 4
A far-red fluorescent protein evolved from a cyanobacterial phycobiliprotein
journal, August 2016
- Rodriguez, Erik A.; Tran, Geraldine N.; Gross, Larry A.
- Nature Methods, Vol. 13, Issue 9
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal