Mechanistic Insights Evaluating Ag, Pb, and Ni as Electrocatalysts for Furfural Reduction from First-Principles Methods
Abstract
Electrochemical reduction of furfural (ECRFF) emerges as an efficient and sustainable means to obtain high-value chemicals and biofuels with the activity and product selectivity being sensitive to cathode materials. In this work, elementary steps describing furfuryl alcohol (FA) and 2-methylfuran (MF) production routes in ECRFF are studied by using periodic density functional theory on Ag, Pb, and Ni, as alternative cathode materials. The established Brønsted–Evans–Polanyi (BEP) relationship has been proven to be reliable to estimate energy barriers of C–O bond cleavage. The intrinsic characters of Ag, Pb, and Ni are then summarized in free energy diagrams to reflect the FA and MF production trends as future guidelines to evaluate ECRFF activity and selectivity on these metals. On all metal surfaces, at both terrace and stepped sites, the first C–H or O–H hydrogenation step, producing respective mh6 or mh7 intermediates, influences overall FA production. On Ag and Pb, pathways involving the mh6 intermediate are thermodynamically and kinetically favored, whereas on Ni, both mh6 and mh7 routes are competitive due to strong interactions between the furan ring and the substrate. In addition, these partially hydrogenated intermediates can also undergo C–O bond cleavage with reduced energy barriers (compared to direct C–O bond cleavagemore »
- Authors:
-
- Kansas State Univ., Manhattan, KS (United States). Dept. of Chemical Engineering
- Univ. of Rochester, NY (United States). Dept. of Chemical Engineering
- Publication Date:
- Research Org.:
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). National Energy Research Scientific Computing Center (NERSC)
- Sponsoring Org.:
- USDOE Office of Science (SC)
- OSTI Identifier:
- 1482388
- Grant/Contract Number:
- AC02-05CH11231; AC02-06CH11357
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Physical Chemistry. C
- Additional Journal Information:
- Journal Volume: 121; Journal Issue: 46; Journal ID: ISSN 1932-7447
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Shan, Nannan, Hanchett, Mary K., and Liu, Bin. Mechanistic Insights Evaluating Ag, Pb, and Ni as Electrocatalysts for Furfural Reduction from First-Principles Methods. United States: N. p., 2017.
Web. doi:10.1021/acs.jpcc.7b06778.
Shan, Nannan, Hanchett, Mary K., & Liu, Bin. Mechanistic Insights Evaluating Ag, Pb, and Ni as Electrocatalysts for Furfural Reduction from First-Principles Methods. United States. https://doi.org/10.1021/acs.jpcc.7b06778
Shan, Nannan, Hanchett, Mary K., and Liu, Bin. Tue .
"Mechanistic Insights Evaluating Ag, Pb, and Ni as Electrocatalysts for Furfural Reduction from First-Principles Methods". United States. https://doi.org/10.1021/acs.jpcc.7b06778. https://www.osti.gov/servlets/purl/1482388.
@article{osti_1482388,
title = {Mechanistic Insights Evaluating Ag, Pb, and Ni as Electrocatalysts for Furfural Reduction from First-Principles Methods},
author = {Shan, Nannan and Hanchett, Mary K. and Liu, Bin},
abstractNote = {Electrochemical reduction of furfural (ECRFF) emerges as an efficient and sustainable means to obtain high-value chemicals and biofuels with the activity and product selectivity being sensitive to cathode materials. In this work, elementary steps describing furfuryl alcohol (FA) and 2-methylfuran (MF) production routes in ECRFF are studied by using periodic density functional theory on Ag, Pb, and Ni, as alternative cathode materials. The established Brønsted–Evans–Polanyi (BEP) relationship has been proven to be reliable to estimate energy barriers of C–O bond cleavage. The intrinsic characters of Ag, Pb, and Ni are then summarized in free energy diagrams to reflect the FA and MF production trends as future guidelines to evaluate ECRFF activity and selectivity on these metals. On all metal surfaces, at both terrace and stepped sites, the first C–H or O–H hydrogenation step, producing respective mh6 or mh7 intermediates, influences overall FA production. On Ag and Pb, pathways involving the mh6 intermediate are thermodynamically and kinetically favored, whereas on Ni, both mh6 and mh7 routes are competitive due to strong interactions between the furan ring and the substrate. In addition, these partially hydrogenated intermediates can also undergo C–O bond cleavage with reduced energy barriers (compared to direct C–O bond cleavage in furfural), which opens potential paths for parallel MF production. Conversion of FA into MF catalyzed by these metallic cathodes was considered as well, although the high C–O bond cleavage energy barrier is likely to hinder this process.},
doi = {10.1021/acs.jpcc.7b06778},
journal = {Journal of Physical Chemistry. C},
number = 46,
volume = 121,
place = {United States},
year = {Tue Oct 24 00:00:00 EDT 2017},
month = {Tue Oct 24 00:00:00 EDT 2017}
}
Web of Science
Figures / Tables:
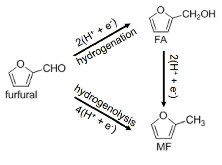 Scheme 1: Routes for Electrochemical Reduction of Furfural (ECRFF) to Furfuryl Alcohol (FA) and 2-methylfuran (MF).
Scheme 1: Routes for Electrochemical Reduction of Furfural (ECRFF) to Furfuryl Alcohol (FA) and 2-methylfuran (MF).
Works referenced in this record:
Furfural: A Promising Platform Compound for Sustainable Production of C 4 and C 5 Chemicals
journal, October 2016
- Li, Xiaodan; Jia, Pei; Wang, Tiefeng
- ACS Catalysis, Vol. 6, Issue 11
Furfural: a renewable and versatile platform molecule for the synthesis of chemicals and fuels
journal, January 2016
- Mariscal, R.; Maireles-Torres, P.; Ojeda, M.
- Energy & Environmental Science, Vol. 9, Issue 4
Furfural-A Promising Platform for Lignocellulosic Biofuels
journal, December 2011
- Lange, Jean-Paul; van der Heide, Evert; van Buijtenen, Jeroen
- ChemSusChem, Vol. 5, Issue 1
Synthesis of Transportation Fuels from Biomass: Chemistry, Catalysts, and Engineering
journal, September 2006
- Huber, George W.; Iborra, Sara; Corma, Avelino
- Chemical Reviews, Vol. 106, Issue 9, p. 4044-4098
Electrochemistry for biofuel generation: production of furans by electrocatalytic hydrogenation of furfurals
journal, January 2013
- Nilges, Peter; Schröder, Uwe
- Energy & Environmental Science, Vol. 6, Issue 10, p. 2925-2931
Electrocatalytic Hydrogenation and Hydrogenolysis of Furfural and the Impact of Homogeneous Side Reactions of Furanic Compounds in Acidic Electrolytes
journal, October 2016
- Jung, Sungyup; Biddinger, Elizabeth J.
- ACS Sustainable Chemistry & Engineering, Vol. 4, Issue 12
Performance of Cu/TiO 2 -SiO 2 catalysts in hydrogenation of furfural to furfuryl alcohol
journal, May 2016
- Li, Feng; Cao, Bo; Ma, Rui
- The Canadian Journal of Chemical Engineering, Vol. 94, Issue 7
Highly efficient nitrogen-doped hierarchically porous carbon supported Ni nanoparticles for the selective hydrogenation of furfural to furfuryl alcohol
journal, January 2016
- Kotbagi, Trupti V.; Gurav, Hanmant R.; Nagpure, Atul S.
- RSC Advances, Vol. 6, Issue 72
Production of Methane and Ethylene in Electrochemical Reduction of Carbon Dioxide at Copper Electrode in Aqueous Hydrogencarbonate Solution
journal, June 1986
- Hori, Yoshio; Kikuchi, Katsuhei; Murata, Akira
- Chemistry Letters, Vol. 15, Issue 6
Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media
journal, August 1994
- Hori, Yoshio; Wakebe, Hidetoshi; Tsukamoto, Toshio
- Electrochimica Acta, Vol. 39, Issue 11-12, p. 1833-1839
Design of Alloy Electrocatalysts for CO[sub 2] Reduction
journal, January 1991
- Watanabe, Masahiro
- Journal of The Electrochemical Society, Vol. 138, Issue 11
Activity Descriptors for CO 2 Electroreduction to Methane on Transition-Metal Catalysts
journal, January 2012
- Peterson, Andrew A.; Nørskov, Jens K.
- The Journal of Physical Chemistry Letters, Vol. 3, Issue 2
Monodisperse Au Nanoparticles for Selective Electrocatalytic Reduction of CO 2 to CO
journal, October 2013
- Zhu, Wenlei; Michalsky, Ronald; Metin, Önder
- Journal of the American Chemical Society, Vol. 135, Issue 45
Mechanistic Insights into the Electrochemical Reduction of CO 2 to CO on Nanostructured Ag Surfaces
journal, June 2015
- Rosen, Jonathan; Hutchings, Gregory S.; Lu, Qi
- ACS Catalysis, Vol. 5, Issue 7
Electrochemical Reduction of Carbon Dioxide to Formate on Tin–Lead Alloys
journal, December 2015
- Choi, Song Yi; Jeong, Soon Kwan; Kim, Hak Joo
- ACS Sustainable Chemistry & Engineering, Vol. 4, Issue 3
Controlling H + vs CO 2 Reduction Selectivity on Pb Electrodes
journal, November 2014
- Lee, Chang Hoon; Kanan, Matthew W.
- ACS Catalysis, Vol. 5, Issue 1
Electrochemical Reduction of Carbon Dioxide on Various Metal Electrodes in Low-Temperature Aqueous KHCO3 Media
journal, January 1990
- Azuma, Masashi; Hashimoto, Kazuhito; Hiramoto, Masahiro
- Journal of The Electrochemical Society, Vol. 137, Issue 6, p. 1772-1778
The Electrochemical Reduction of Furfural
journal, January 1939
- Albert, William C.; Lowy, Alexander
- Transactions of The Electrochemical Society, Vol. 75, Issue 1
Electrochemical investigations of the oxidation–reduction of furfural in aqueous medium
journal, January 2004
- Parpot, P.; Bettencourt, A. P.; Chamoulaud, G.
- Electrochimica Acta, Vol. 49, Issue 3, p. 397-403
Aqueous electrocatalytic hydrogenation of furfural using a sacrificial anode
journal, March 2012
- Li, Zhenglong; Kelkar, Shantanu; Lam, Chun Ho
- Electrochimica Acta, Vol. 64
Electrocatalytic hydrogenation of furfural to furfuryl alcohol using platinum supported on activated carbon fibers
journal, July 2014
- zhao, Bo; Chen, Mengyuan; Guo, Qingxiang
- Electrochimica Acta, Vol. 135
The electrocatalytic hydrogenation of furanic compounds in a continuous electrocatalytic membrane reactor
journal, January 2013
- Green, Sara K.; Lee, Jechan; Kim, Hyung Ju
- Green Chemistry, Vol. 15, Issue 7, p. 1869-1879
Fabrication of La-doped TiO2 Film Electrode and investigation of its electrocatalytic activity for furfural reduction
journal, January 2015
- Wang, Fengwu; Xu, Mai; Wei, Lin
- Electrochimica Acta, Vol. 153
Effects of van der Waals density functional corrections on trends in furfural adsorption and hydrogenation on close-packed transition metal surfaces
journal, April 2014
- Liu, Bin; Cheng, Lei; Curtiss, Larry
- Surface Science, Vol. 622
Exploring Furfural Catalytic Conversion on Cu(111) from Computation
journal, June 2015
- Shi, Yun; Zhu, Yulei; Yang, Yong
- ACS Catalysis, Vol. 5, Issue 7
DFT Study of Furfural Conversion to Furan, Furfuryl Alcohol, and 2-Methylfuran on Pd(111)
journal, October 2012
- Vorotnikov, Vassili; Mpourmpakis, Giannis; Vlachos, Dionisios G.
- ACS Catalysis, Vol. 2, Issue 12
Reaction pathways of furfural, furfuryl alcohol and 2-methylfuran on Cu(111) and NiCu bimetallic surfaces
journal, October 2016
- Xiong, Ke; Wan, Weiming; Chen, Jingguang G.
- Surface Science, Vol. 652
Adsorption and Reaction of Furfural and Furfuryl Alcohol on Pd(111): Unique Reaction Pathways for Multifunctional Reagents
journal, August 2011
- Pang, Simon H.; Medlin, J. Will
- ACS Catalysis, Vol. 1, Issue 10
Selective conversion of furfural to methylfuran over silica-supported NiFe bimetallic catalysts
journal, November 2011
- Sitthisa, Surapas; An, Wei; Resasco, Daniel E.
- Journal of Catalysis, Vol. 284, Issue 1
Conversion of furfural and 2-methylpentanal on Pd/SiO2 and Pd–Cu/SiO2 catalysts
journal, May 2011
- Sitthisa, Surapas; Pham, Trung; Prasomsri, Teerawit
- Journal of Catalysis, Vol. 280, Issue 1
Kinetics and mechanism of hydrogenation of furfural on Cu/SiO2 catalysts
journal, January 2011
- Sitthisa, Surapas; Sooknoi, Tawan; Ma, Yuguang
- Journal of Catalysis, Vol. 277, Issue 1
Ring‐Opening Reaction of Furfural and Tetrahydrofurfuryl Alcohol on Hydrogen‐Predosed Iridium(1 1 1) and Cobalt/Iridium(1 1 1) Surfaces
journal, March 2017
- Wan, Weiming; Jenness, Glen R.; Xiong, Ke
- ChemCatChem, Vol. 9, Issue 9
Efficient and Selective Electrochemical and Photoelectrochemical Reduction of 5-Hydroxymethylfurfural to 2,5-Bis(hydroxymethyl)furan using Water as the Hydrogen Source
journal, February 2016
- Roylance, John J.; Kim, Tae Woo; Choi, Kyoung-Shin
- ACS Catalysis, Vol. 6, Issue 3
Ab initio molecular dynamics for open-shell transition metals
journal, November 1993
- Kresse, G.; Hafner, J.
- Physical Review B, Vol. 48, Issue 17
Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set
journal, July 1996
- Kresse, G.; Furthmüller, J.
- Computational Materials Science, Vol. 6, Issue 1, p. 15-50
Generalized Gradient Approximation Made Simple
journal, October 1996
- Perdew, John P.; Burke, Kieron; Ernzerhof, Matthias
- Physical Review Letters, Vol. 77, Issue 18, p. 3865-3868
Special points for Brillouin-zone integrations
journal, June 1976
- Monkhorst, Hendrik J.; Pack, James D.
- Physical Review B, Vol. 13, Issue 12, p. 5188-5192
High-precision sampling for Brillouin-zone integration in metals
journal, August 1989
- Methfessel, M.; Paxton, A. T.
- Physical Review B, Vol. 40, Issue 6
Practical principles of density functional theory for catalytic reaction simulations on metal surfaces – from theory to applications
journal, March 2017
- Shan, Nannan; Zhou, Mingxia; Hanchett, Mary K.
- Molecular Simulation, Vol. 43, Issue 10-11
Adsorption of small aromatic molecules on the (111) surfaces of noble metals: A density functional theory study with semiempirical corrections for dispersion effects
journal, June 2010
- Tonigold, Katrin; Groß, Axel
- The Journal of Chemical Physics, Vol. 132, Issue 22
Adsorption of Benzene on Copper, Silver, and Gold Surfaces
journal, May 2006
- Bilić, Ante; Reimers, Jeffrey R.; Hush, Noel S.
- Journal of Chemical Theory and Computation, Vol. 2, Issue 4
Semiempirical GGA-type density functional constructed with a long-range dispersion correction
journal, January 2006
- Grimme, Stefan
- Journal of Computational Chemistry, Vol. 27, Issue 15, p. 1787-1799
Chemical accuracy for the van der Waals density functional
journal, December 2009
- Klimeš, Jiří; Bowler, David R.; Michaelides, Angelos
- Journal of Physics: Condensed Matter, Vol. 22, Issue 2
SurfKin: An ab initio kinetic code for modeling surface reactions
journal, August 2014
- Le, Thong Nguyen-Minh; Liu, Bin; Huynh, Lam K.
- Journal of Computational Chemistry, Vol. 35, Issue 26
Origin of the Overpotential for Oxygen Reduction at a Fuel-Cell Cathode
journal, November 2004
- Nørskov, J. K.; Rossmeisl, J.; Logadottir, A.
- The Journal of Physical Chemistry B, Vol. 108, Issue 46
A climbing image nudged elastic band method for finding saddle points and minimum energy paths
journal, December 2000
- Henkelman, Graeme; Uberuaga, Blas P.; Jónsson, Hannes
- The Journal of Chemical Physics, Vol. 113, Issue 22, p. 9901-9904
A dimer method for finding saddle points on high dimensional potential surfaces using only first derivatives
journal, October 1999
- Henkelman, Graeme; Jónsson, Hannes
- The Journal of Chemical Physics, Vol. 111, Issue 15
Decomposition Pathways of Glycerol via C–H, O–H, and C–C Bond Scission on Pt(111): A Density Functional Theory Study
journal, September 2011
- Liu, Bin; Greeley, Jeffrey
- The Journal of Physical Chemistry C, Vol. 115, Issue 40
Universality in Oxygen Evolution Electrocatalysis on Oxide Surfaces
journal, March 2011
- Man, Isabela C.; Su, Hai‐Yan; Calle‐Vallejo, Federico
- ChemCatChem, Vol. 3, Issue 7
On the pH dependence of electrochemical proton transfer barriers
journal, March 2016
- Rossmeisl, Jan; Chan, Karen; Skúlason, Egill
- Catalysis Today, Vol. 262
Electroreduction of N 2 to Ammonia at Ambient Conditions on Mononitrides of Zr, Nb, Cr, and V: A DFT Guide for Experiments
journal, December 2015
- Abghoui, Younes; Garden, Anna L.; Howalt, Jakob G.
- ACS Catalysis, Vol. 6, Issue 2
Free-Energy Barriers and Reaction Mechanisms for the Electrochemical Reduction of CO on the Cu(100) Surface, Including Multiple Layers of Explicit Solvent at pH 0
journal, November 2015
- Cheng, Tao; Xiao, Hai; Goddard, William A.
- The Journal of Physical Chemistry Letters, Vol. 6, Issue 23
Works referencing / citing this record:
The selective hydrogenation of furfural over intermetallic compounds with outstanding catalytic performance
journal, January 2019
- Yang, Yusen; Chen, Lifang; Chen, Yudi
- Green Chemistry, Vol. 21, Issue 19

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal