Rechargeable Aqueous Zn2+-Battery with High Power Density and Long Cycle-life
Abstract
Li-ion batteries (LIBs) are approaching their energy limits imposed by the intercalation chemistry nature. As alternatives, multivalent (MV) chemistries bring both promises and challenges, with the main obstacle being the sluggish diffusion of MV-cations due to their strong electrostatic interaction with host lattices. In this work, we demonstrated that polyanion based robust crystal architecture could enable the ultrafast and reversible Zn2+-intercalation and de-intercalation at a high working voltage. The nominal bivalence of Zn2+ was successfully delocalized by the multiple atoms through the p-d hybridization between the V-d and O-p orbitals, hence the inserted Zn2+ only bears an effective charge of 1.336, rendering its high mobility. The novel aqueous rechargeable 1.7 V Zn/LiV2(PO4)3 cell based on such mechanism delivers a high power density (8000 W/kg at 60 C) comparable to supercapacitors, a high energy density (218 Wh/Kg at 1 C) close to LIBs, with extraordinary long cycle life of 4000 cycles. All of these parameters far exceed any Zn battery reported so far. The cell-level volumetric and specific energy densities of the Zn/LiV2(PO4)3 cell are 320 Wh/L and 150 Wh/kg, respectively, which are even better than the first-generation LIBs. Furthermore, combining with the intrinsic safety of the aqueous chemistry and themore »
- Authors:
-
- Univ. of Maryland, College Park, MD (United States); U.S. Army Research Lab., Adelphi, MD (United States)
- Brookhaven National Lab. (BNL), Upton, NY (United States)
- Univ. of Maryland, College Park, MD (United States)
- U.S. Army Research Lab., Adelphi, MD (United States)
- Publication Date:
- Research Org.:
- Brookhaven National Laboratory (BNL), Upton, NY (United States)
- Sponsoring Org.:
- USDOE Office of Energy Efficiency and Renewable Energy (EERE), Vehicle Technologies Office (EE-3V); USDOE Advanced Research Projects Agency - Energy (ARPA-E)
- OSTI Identifier:
- 1476764
- Alternate Identifier(s):
- OSTI ID: 1477130
- Report Number(s):
- BNL-209154-2018-JAAM
Journal ID: ISSN 1754-5692
- Grant/Contract Number:
- SC0012704; AR0000389
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Energy & Environmental Science
- Additional Journal Information:
- Journal Volume: 11; Journal Issue: 11; Journal ID: ISSN 1754-5692
- Publisher:
- Royal Society of Chemistry
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 25 ENERGY STORAGE
Citation Formats
Wang, Fei, Hu, Enyuan, Sun, Wei, Gao, Tao, Ji, Xiao, Fan, Xiulin, Han, Fudong, Yang, Xiao -Qing, Xu, Kang, and Wang, Chunsheng. Rechargeable Aqueous Zn2+-Battery with High Power Density and Long Cycle-life. United States: N. p., 2018.
Web. doi:10.1039/C8EE01883A.
Wang, Fei, Hu, Enyuan, Sun, Wei, Gao, Tao, Ji, Xiao, Fan, Xiulin, Han, Fudong, Yang, Xiao -Qing, Xu, Kang, & Wang, Chunsheng. Rechargeable Aqueous Zn2+-Battery with High Power Density and Long Cycle-life. United States. https://doi.org/10.1039/C8EE01883A
Wang, Fei, Hu, Enyuan, Sun, Wei, Gao, Tao, Ji, Xiao, Fan, Xiulin, Han, Fudong, Yang, Xiao -Qing, Xu, Kang, and Wang, Chunsheng. Wed .
"Rechargeable Aqueous Zn2+-Battery with High Power Density and Long Cycle-life". United States. https://doi.org/10.1039/C8EE01883A. https://www.osti.gov/servlets/purl/1476764.
@article{osti_1476764,
title = {Rechargeable Aqueous Zn2+-Battery with High Power Density and Long Cycle-life},
author = {Wang, Fei and Hu, Enyuan and Sun, Wei and Gao, Tao and Ji, Xiao and Fan, Xiulin and Han, Fudong and Yang, Xiao -Qing and Xu, Kang and Wang, Chunsheng},
abstractNote = {Li-ion batteries (LIBs) are approaching their energy limits imposed by the intercalation chemistry nature. As alternatives, multivalent (MV) chemistries bring both promises and challenges, with the main obstacle being the sluggish diffusion of MV-cations due to their strong electrostatic interaction with host lattices. In this work, we demonstrated that polyanion based robust crystal architecture could enable the ultrafast and reversible Zn2+-intercalation and de-intercalation at a high working voltage. The nominal bivalence of Zn2+ was successfully delocalized by the multiple atoms through the p-d hybridization between the V-d and O-p orbitals, hence the inserted Zn2+ only bears an effective charge of 1.336, rendering its high mobility. The novel aqueous rechargeable 1.7 V Zn/LiV2(PO4)3 cell based on such mechanism delivers a high power density (8000 W/kg at 60 C) comparable to supercapacitors, a high energy density (218 Wh/Kg at 1 C) close to LIBs, with extraordinary long cycle life of 4000 cycles. All of these parameters far exceed any Zn battery reported so far. The cell-level volumetric and specific energy densities of the Zn/LiV2(PO4)3 cell are 320 Wh/L and 150 Wh/kg, respectively, which are even better than the first-generation LIBs. Furthermore, combining with the intrinsic safety of the aqueous chemistry and the wide working temperature range, this cell makes a strong candidate for automotive applications.},
doi = {10.1039/C8EE01883A},
journal = {Energy & Environmental Science},
number = 11,
volume = 11,
place = {United States},
year = {Wed Oct 03 00:00:00 EDT 2018},
month = {Wed Oct 03 00:00:00 EDT 2018}
}
Web of Science
Figures / Tables:
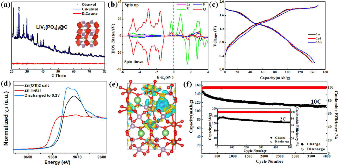 Figure 1: Structure of LiV2(PO4)3 and its electrochemical behavior. (a) The XRD pattern and its Rietveld refinement results of the LiV2(PO4)3@C prepared through the electrochemical delithiation process. (b) The partial density of states (DOS) for LiV2(PO4)3. (c) The typical voltage profile of Zn/LiV2(PO4)3 cell between 0.2 V and 1.9 Vmore »
Figure 1: Structure of LiV2(PO4)3 and its electrochemical behavior. (a) The XRD pattern and its Rietveld refinement results of the LiV2(PO4)3@C prepared through the electrochemical delithiation process. (b) The partial density of states (DOS) for LiV2(PO4)3. (c) The typical voltage profile of Zn/LiV2(PO4)3 cell between 0.2 V and 1.9 Vmore »
Works referenced in this record:
Layered VS 2 Nanosheet-Based Aqueous Zn Ion Battery Cathode
journal, January 2017
- He, Pan; Yan, Mengyu; Zhang, Guobin
- Advanced Energy Materials, Vol. 7, Issue 11
Comparison of electrochemical performances of olivine NaFePO 4 in sodium-ion batteries and olivine LiFePO 4 in lithium-ion batteries
journal, January 2013
- Zhu, Yujie; Xu, Yunhua; Liu, Yihang
- Nanoscale, Vol. 5, Issue 2
Rhombohedral Form of Li 3 V 2 (PO 4 ) 3 as a Cathode in Li-Ion Batteries
journal, November 2000
- Gaubicher, J.; Wurm, C.; Goward, G.
- Chemistry of Materials, Vol. 12, Issue 11
Towards polyvalent ion batteries: A zinc-ion battery based on NASICON structured Na3V2(PO4)3
journal, July 2016
- Li, Guolong; Yang, Ze; Jiang, Yan
- Nano Energy, Vol. 25
Challenges for Rechargeable Li Batteries
journal, February 2010
- Goodenough, John B.; Kim, Youngsik
- Chemistry of Materials, Vol. 22, Issue 3, p. 587-603
LiVPO4F/Li3V2(PO4)3 nanostructured composite cathode materials prepared via mechanochemical way
journal, August 2013
- Kosova, Nina V.; Devyatkina, Evgeniya T.; Slobodyuk, Arseny B.
- Journal of Solid State Electrochemistry, Vol. 18, Issue 5
Materials Design Rules for Multivalent Ion Mobility in Intercalation Structures
journal, August 2015
- Rong, Ziqin; Malik, Rahul; Canepa, Pieremanuele
- Chemistry of Materials, Vol. 27, Issue 17
Electrochemical Zinc Intercalation in Lithium Vanadium Oxide: A High-Capacity Zinc-Ion Battery Cathode
journal, February 2017
- Alfaruqi, Muhammad H.; Mathew, Vinod; Song, Jinju
- Chemistry of Materials, Vol. 29, Issue 4
Cation-Deficient Spinel ZnMn 2 O 4 Cathode in Zn(CF 3 SO 3 ) 2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery
journal, September 2016
- Zhang, Ning; Cheng, Fangyi; Liu, Yongchang
- Journal of the American Chemical Society, Vol. 138, Issue 39
Rechargeable magnesium-ion battery based on a TiSe2-cathode with d-p orbital hybridized electronic structure
journal, July 2015
- Gu, Yunpeng; Katsura, Yukari; Yoshino, Takafumi
- Scientific Reports, Vol. 5, Issue 1
Electrochemical Zinc-Ion Intercalation Properties and Crystal Structures of ZnMo 6 S 8 and Zn 2 Mo 6 S 8 Chevrel Phases in Aqueous Electrolytes
journal, March 2016
- Chae, Munseok S.; Heo, Jongwook W.; Lim, Sung-Chul
- Inorganic Chemistry, Vol. 55, Issue 7
Odyssey of Multivalent Cathode Materials: Open Questions and Future Challenges
journal, February 2017
- Canepa, Pieremanuele; Sai Gautam, Gopalakrishnan; Hannah, Daniel C.
- Chemical Reviews, Vol. 117, Issue 5
Carbon-coated rhombohedral Li 3 V 2 (PO 4 ) 3 as both cathode and anode materials for lithium-ion batteries: electrochemical performance and lithium storage mechanism
journal, January 2014
- Jian, Zelang; Han, Wenze; Liang, Yanliang
- J. Mater. Chem. A, Vol. 2, Issue 47
Electrochemical Property: Structure Relationships in Monoclinic Li 3 - y V 2 (PO 4 ) 3
journal, August 2003
- Yin, S. -C.; Grondey, H.; Strobel, P.
- Journal of the American Chemical Society, Vol. 125, Issue 34
Energetic Zinc Ion Chemistry: The Rechargeable Zinc Ion Battery
journal, December 2011
- Xu, Chengjun; Li, Baohua; Du, Hongda
- Angewandte Chemie International Edition, Vol. 51, Issue 4
Electrochemically Induced Structural Transformation in a γ-MnO 2 Cathode of a High Capacity Zinc-Ion Battery System
journal, May 2015
- Alfaruqi, Muhammad H.; Mathew, Vinod; Gim, Jihyeon
- Chemistry of Materials, Vol. 27, Issue 10
Pyro-synthesis of a high rate nano-Li3V2(PO4)3/C cathode with mixed morphology for advanced Li-ion batteries
journal, February 2014
- Kang, Jungwon; Mathew, Vinod; Gim, Jihyeon
- Scientific Reports, Vol. 4, Issue 1
A better understanding of the capacity fading mechanisms of Li 3 V 2 (PO 4 ) 3
journal, January 2015
- Wang, Liping; Xu, Jin; Wang, Chong
- RSC Advances, Vol. 5, Issue 88
Experimental visualization of lithium diffusion in LixFePO4
journal, August 2008
- Nishimura, Shin-ichi; Kobayashi, Genki; Ohoyama, Kenji
- Nature Materials, Vol. 7, Issue 9
A High Power Rechargeable Nonaqueous Multivalent Zn/V 2 O 5 Battery
journal, August 2016
- Senguttuvan, Premkumar; Han, Sang-Don; Kim, Soojeong
- Advanced Energy Materials, Vol. 6, Issue 24
The electronic structure and band gap of LiFePO4 and LiMnPO4
journal, October 2004
- Zhou, Fei; Kang, Kisuk; Maxisch, Thomas
- Solid State Communications, Vol. 132, Issue 3-4
Todorokite-type MnO2 as a zinc-ion intercalating material
journal, December 2013
- Lee, Jonghyuk; Ju, Jeh Beck; Cho, Won Il
- Electrochimica Acta, Vol. 112
Battery materials for ultrafast charging and discharging
journal, March 2009
- Kang, Byoungwoo; Ceder, Gerbrand
- Nature, Vol. 458, Issue 7235, p. 190-193
Study on structure and electrochemical properties of carbon-coated monoclinic Li3V2(PO4)3 using synchrotron based in situ X-ray diffraction and absorption
journal, August 2013
- Yoon, Jeongbae; Muhammad, Shoaib; Jang, Donghyuk
- Journal of Alloys and Compounds, Vol. 569
Disulfide-Bridged (Mo 3 S 11 ) Cluster Polymer: Molecular Dynamics and Application as Electrode Material for a Rechargeable Magnesium Battery
journal, August 2016
- Truong, Quang Duc; Kempaiah Devaraju, Murukanahally; Nguyen, Duc N.
- Nano Letters, Vol. 16, Issue 9
Nanostructured Composites: A High Capacity, Fast Rate Li3V2(PO4)3/Carbon Cathode for Rechargeable Lithium Batteries
journal, November 2002
- Huang, H.; Yin, S. -C.; Kerr, T.
- Advanced Materials, Vol. 14, Issue 21
High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance
journal, April 2013
- Augustyn, Veronica; Come, Jérémy; Lowe, Michael A.
- Nature Materials, Vol. 12, Issue 6
Building better batteries
journal, February 2008
- Armand, M.; Tarascon, J.-M.
- Nature, Vol. 451, Issue 7179, p. 652-657
Atomistic Simulation Study of Monoclinic Li 3 V 2 (PO 4 ) 3 as a Cathode Material for Lithium Ion Battery: Structure, Defect Chemistry, Lithium Ion Transport Pathway, and Dynamics
journal, November 2012
- Lee, Sanghun; Park, Sung Soo
- The Journal of Physical Chemistry C, Vol. 116, Issue 48
Enhancement of electronic conductivity of by Cr doping and its identification by first-principles calculations
journal, November 2003
- Shi, Siqi; Liu, Lijun; Ouyang, Chuying
- Physical Review B, Vol. 68, Issue 19
"Water-in-salt" electrolyte enables high-voltage aqueous lithium-ion chemistries
journal, November 2015
- Suo, L.; Borodin, O.; Gao, T.
- Science, Vol. 350, Issue 6263
Electrical Energy Storage for the Grid: A Battery of Choices
journal, November 2011
- Dunn, B.; Kamath, H.; Tarascon, J. -M.
- Science, Vol. 334, Issue 6058
Towards High-Voltage Aqueous Metal-Ion Batteries Beyond 1.5 V: The Zinc/Zinc Hexacyanoferrate System
journal, September 2014
- Zhang, Leyuan; Chen, Liang; Zhou, Xufeng
- Advanced Energy Materials, Vol. 5, Issue 2
A high-capacity and long-life aqueous rechargeable zinc battery using a metal oxide intercalation cathode
journal, August 2016
- Kundu, Dipan; Adams, Brian D.; Duffort, Victor
- Nature Energy, Vol. 1, Issue 10
Novel Rechargeable M3V2(PO4)3//Zinc (M = Li, Na) Hybrid Aqueous Batteries with Excellent Cycling Performance
journal, May 2016
- Zhao, H. B.; Hu, C. J.; Cheng, H. W.
- Scientific Reports, Vol. 6, Issue 1
Reversible aqueous zinc/manganese oxide energy storage from conversion reactions
journal, April 2016
- Pan, Huilin; Shao, Yuyan; Yan, Pengfei
- Nature Energy, Vol. 1, Issue 5
Promise and reality of post-lithium-ion batteries with high energy densities
journal, March 2016
- Choi, Jang Wook; Aurbach, Doron
- Nature Reviews Materials, Vol. 1, Issue 4
Highly reversible zinc metal anode for aqueous batteries
journal, April 2018
- Wang, Fei; Borodin, Oleg; Gao, Tao
- Nature Materials, Vol. 17, Issue 6
Rechargeable nickel–3D zinc batteries: An energy-dense, safer alternative to lithium-ion
journal, April 2017
- Parker, Joseph F.; Chervin, Christopher N.; Pala, Irina R.
- Science, Vol. 356, Issue 6336
Zinc-Iron Flow Batteries with Common Electrolyte
journal, January 2017
- Selverston, S.; Savinell, R. F.; Wainright, J. S.
- Journal of The Electrochemical Society, Vol. 164, Issue 6
Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte
journal, August 2010
- Luo, Jia-Yan; Cui, Wang-Jun; He, Ping
- Nature Chemistry, Vol. 2, Issue 9
Nanostructured Composites: A High Capacity, Fast Rate Li3V2(PO4)3/Carbon Cathode for Rechargeable Lithium Batteries.
journal, January 2003
- Huang, Huan; Yin, Shieh-Chieh; Kerr, Tracy
- ChemInform, Vol. 34, Issue 3
Electrochemical Property: Structure Relationships in Monoclinic Li3-yV2(PO4)3.
journal, November 2003
- Yin, S. -C.; Grondey, H.; Strobel, P.
- ChemInform, Vol. 34, Issue 46
The electronic structure and band gap of LiFePO4 and LiMnPO4
text, January 2005
- Zhou, Fei; Kang, Kisuk; Maxisch, Thomas
- arXiv
Works referencing / citing this record:
ZnCl 2 “Water‐in‐Salt” Electrolyte Transforms the Performance of Vanadium Oxide as a Zn Battery Cathode
journal, May 2019
- Zhang, Lu; Rodríguez‐Pérez, Ismael A.; Jiang, Heng
- Advanced Functional Materials, Vol. 29, Issue 30
Toward High‐Performance Hybrid Zn‐Based Batteries via Deeply Understanding Their Mechanism and Using Electrolyte Additive
journal, June 2019
- Hao, Junnan; Long, Jun; Li, Bo
- Advanced Functional Materials, Vol. 29, Issue 34
Electronic Structure Regulation of Layered Vanadium Oxide via Interlayer Doping Strategy toward Superior High‐Rate and Low‐Temperature Zinc‐Ion Batteries
journal, November 2019
- Geng, Hongbo; Cheng, Min; Wang, Bo
- Advanced Functional Materials, Vol. 30, Issue 6
Recent Advances and Prospects of Cathode Materials for Rechargeable Aqueous Zinc‐Ion Batteries
journal, July 2019
- Chen, Lineng; An, Qinyou; Mai, Liqiang
- Advanced Materials Interfaces, Vol. 6, Issue 17
A Room‐Temperature Molten Hydrate Electrolyte for Rechargeable Zinc–Air Batteries
journal, April 2019
- Chen, Chih‐Yao; Matsumoto, Kazuhiko; Kubota, Keigo
- Advanced Energy Materials, Vol. 9, Issue 22
An Ultrastable Presodiated Titanium Disulfide Anode for Aqueous “Rocking‐Chair” Zinc Ion Battery
journal, June 2019
- Li, Wei; Wang, Kangli; Cheng, Shijie
- Advanced Energy Materials, Vol. 9, Issue 27
A Flexible Solid‐State Aqueous Zinc Hybrid Battery with Flat and High‐Voltage Discharge Plateau
journal, October 2019
- Liu, Zhuoxin; Yang, Qi; Wang, Donghong
- Advanced Energy Materials, Vol. 9, Issue 46
Inhibiting VOPO 4 ⋅ x H 2 O Decomposition and Dissolution in Rechargeable Aqueous Zinc Batteries to Promote Voltage and Capacity Stabilities
journal, November 2019
- Shi, Hua‐Yu; Song, Yu; Qin, Zengming
- Angewandte Chemie International Edition, Vol. 58, Issue 45
Mn 2 O 3 /Al 2 O 3 cathode material derived from a metal–organic framework with enhanced cycling performance for aqueous zinc-ion batteries
journal, January 2020
- Gou, Lei; Mou, Ke-Liang; Fan, Xiao-Yong
- Dalton Transactions, Vol. 49, Issue 3
Expanded hydrated vanadate for high-performance aqueous zinc-ion batteries
journal, January 2019
- Liu, Chaofeng; Neale, Zachary; Zheng, Jiqi
- Energy & Environmental Science, Vol. 12, Issue 7
Issues and opportunities facing aqueous zinc-ion batteries
journal, January 2019
- Tang, Boya; Shan, Lutong; Liang, Shuquan
- Energy & Environmental Science, Vol. 12, Issue 11
Rod-like anhydrous V 2 O 5 assembled by tiny nanosheets as a high-performance cathode material for aqueous zinc-ion batteries
journal, January 2019
- Zhou, Weijun; Chen, Jizhang; Chen, Minfeng
- RSC Advances, Vol. 9, Issue 52
Anchoring V 2 O 5 nanosheets on hierarchical titanium nitride nanowire arrays to form core–shell heterostructures as a superior cathode for high-performance wearable aqueous rechargeable zinc-ion batteries
journal, January 2019
- Li, Qiulong; Zhang, Qichong; Liu, Chenglong
- Journal of Materials Chemistry A, Vol. 7, Issue 21
A high energy efficiency and long life aqueous Zn–I 2 battery
journal, January 2020
- Li, Wei; Wang, Kangli; Jiang, Kai
- Journal of Materials Chemistry A, Vol. 8, Issue 7
Inhibiting VOPO 4 ⋅ x H 2 O Decomposition and Dissolution in Rechargeable Aqueous Zinc Batteries to Promote Voltage and Capacity Stabilities
journal, September 2019
- Shi, Hua‐Yu; Song, Yu; Qin, Zengming
- Angewandte Chemie, Vol. 131, Issue 45
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal