Reinterpreting the infrared spectrum of H + HCN: Methylene amidogen radical and its coproducts
Abstract
The methylene amidogen radical (H2CN) plays a role in high-energy material combustion and extraterresterial atmospheres. Recent theoretical work has struggled to match experimental assignments for its CN and antisymmetric CH2 stretching frequencies (ν2 and ν5), which were reported to occur at 1725 and 3103 cm–1. Herein, we compute the vibrational energy levels of this molecule by extrapolating quadruples-level coupled- cluster theory to the complete basis limit and adding corrections for vibrational anharmonicity. This level of theory predicts that ν2 and ν5 should occur at 1646 and 2892 cm–1, at odds with the experimental assignments. To investigate the possibility of defects in our theoretical treatment, we analyze the sensitivity of our approach to each of its contributing approximations. Our analysis suggests that the observed deviation from experiment is too large to be explained as an accumulation of errors, leading us to conclude that these transitions were misassigned. To help resolve this discrepancy, we investigate possible byproducts of the H + HCN reaction, which was the source of H2CN in the original experiment. In particular, we predict vibrational spectra for cis-HCNH, trans-HCNH, and H2CNH using high-level coupled-cluster computations. Based on these results, we reassign the transition at 1725 cm–1 to ν3 ofmore »
- Authors:
-
- Univ. of Georgia, Athens, GA (United States)
- Univ. of Georgia, Athens, GA (United States); Messiah College, Mechanicsburg, PA (United States)
- Univ. of Georgia, Athens, GA (United States); Federal Univ. of Sao Paulo, Sao Paulo (Brazil)
- Univ. of Georgia, Athens, GA (United States); Biola Univ., La Mirada, CA (United States)
- Publication Date:
- Research Org.:
- Univ. of Georgia, Athens, GA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1468296
- Alternate Identifier(s):
- OSTI ID: 1415508
- Grant/Contract Number:
- SC0018412; SC0015512
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Chemical Physics
- Additional Journal Information:
- Journal Volume: 148; Journal Issue: 1; Journal ID: ISSN 0021-9606
- Publisher:
- American Institute of Physics (AIP)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 71 CLASSICAL AND QUANTUM MECHANICS, GENERAL PHYSICS
Citation Formats
Wiens, Avery E., Copan, Andreas V., Rossomme, Elliot C., Aroeira, Gustavo J. R., Bernstein, Olivia M., Agarwal, Jay, and Schaefer, Henry F. Reinterpreting the infrared spectrum of H + HCN: Methylene amidogen radical and its coproducts. United States: N. p., 2018.
Web. doi:10.1063/1.5004984.
Wiens, Avery E., Copan, Andreas V., Rossomme, Elliot C., Aroeira, Gustavo J. R., Bernstein, Olivia M., Agarwal, Jay, & Schaefer, Henry F. Reinterpreting the infrared spectrum of H + HCN: Methylene amidogen radical and its coproducts. United States. https://doi.org/10.1063/1.5004984
Wiens, Avery E., Copan, Andreas V., Rossomme, Elliot C., Aroeira, Gustavo J. R., Bernstein, Olivia M., Agarwal, Jay, and Schaefer, Henry F. Wed .
"Reinterpreting the infrared spectrum of H + HCN: Methylene amidogen radical and its coproducts". United States. https://doi.org/10.1063/1.5004984. https://www.osti.gov/servlets/purl/1468296.
@article{osti_1468296,
title = {Reinterpreting the infrared spectrum of H + HCN: Methylene amidogen radical and its coproducts},
author = {Wiens, Avery E. and Copan, Andreas V. and Rossomme, Elliot C. and Aroeira, Gustavo J. R. and Bernstein, Olivia M. and Agarwal, Jay and Schaefer, Henry F.},
abstractNote = {The methylene amidogen radical (H2CN) plays a role in high-energy material combustion and extraterresterial atmospheres. Recent theoretical work has struggled to match experimental assignments for its CN and antisymmetric CH2 stretching frequencies (ν2 and ν5), which were reported to occur at 1725 and 3103 cm–1. Herein, we compute the vibrational energy levels of this molecule by extrapolating quadruples-level coupled- cluster theory to the complete basis limit and adding corrections for vibrational anharmonicity. This level of theory predicts that ν2 and ν5 should occur at 1646 and 2892 cm–1, at odds with the experimental assignments. To investigate the possibility of defects in our theoretical treatment, we analyze the sensitivity of our approach to each of its contributing approximations. Our analysis suggests that the observed deviation from experiment is too large to be explained as an accumulation of errors, leading us to conclude that these transitions were misassigned. To help resolve this discrepancy, we investigate possible byproducts of the H + HCN reaction, which was the source of H2CN in the original experiment. In particular, we predict vibrational spectra for cis-HCNH, trans-HCNH, and H2CNH using high-level coupled-cluster computations. Based on these results, we reassign the transition at 1725 cm–1 to ν3 of trans-HCNH, yielding excellent agreement. Supporting this identification, we assign a known contaminant peak at 886 cm–1 to ν5 of the same conformer. Our computations suggest that the peak observed at 3103 cm–1, however, does not belong to any of the aforementioned species. To facilitate further investigation, we use structure and bonding arguments to narrow the range of possible candidates. Furthermore, these arguments lead us to tentatively put forth formaldazine [(H2CN)2] as a suggestion for further study, which we support with additional computations.},
doi = {10.1063/1.5004984},
journal = {Journal of Chemical Physics},
number = 1,
volume = 148,
place = {United States},
year = {Wed Jan 03 00:00:00 EST 2018},
month = {Wed Jan 03 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
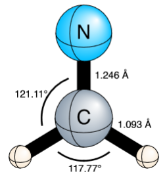 FIG. 1.: Planar equilibrium molecular structure for the ground (X̃ 2/B2) electronic state of H2CN, optimized at the CCSDT(Q)/CBS level of theory.
FIG. 1.: Planar equilibrium molecular structure for the ground (X̃ 2/B2) electronic state of H2CN, optimized at the CCSDT(Q)/CBS level of theory.
Works referenced in this record:
Psi4 1.1: An Open-Source Electronic Structure Program Emphasizing Automation, Advanced Libraries, and Interoperability
journal, June 2017
- Parrish, Robert M.; Burns, Lori A.; Smith, Daniel G. A.
- Journal of Chemical Theory and Computation, Vol. 13, Issue 7
Analytic energy gradients for the spin-free exact two-component theory using an exact block diagonalization for the one-electron Dirac Hamiltonian
journal, August 2011
- Cheng, Lan; Gauss, Jürgen
- The Journal of Chemical Physics, Vol. 135, Issue 8
Nitramine propellant ignition and combustion research
journal, January 1991
- Alexander, M. H.; Dagdigian, P. J.; Jacox, M. E.
- Progress in Energy and Combustion Science, Vol. 17, Issue 4
Representing Global Reactive Potential Energy Surfaces Using Gaussian Processes
journal, March 2017
- Kolb, Brian; Marshall, Paul; Zhao, Bin
- The Journal of Physical Chemistry A, Vol. 121, Issue 13
The role of accurate quantum mechanical computations in the assignment of vibrational spectra for unstable free radicals: H2CN and F2CN as test cases
journal, January 2009
- Puzzarini, Cristina; Barone, Vincenzo
- Chemical Physics Letters, Vol. 467, Issue 4-6
Molpro: a general-purpose quantum chemistry program package: Molpro
journal, July 2011
- Werner, Hans-Joachim; Knowles, Peter J.; Knizia, Gerald
- Wiley Interdisciplinary Reviews: Computational Molecular Science, Vol. 2, Issue 2
The Vibration-Rotation Energies of Molecules
journal, April 1951
- Nielsen, Harald H.
- Reviews of Modern Physics, Vol. 23, Issue 2
HCN formation on Jupiter: The coupled photochemistry of ammonia and acetylene
journal, June 1983
- Kaye, Jack A.; Strobel, Darrell F.
- Icarus, Vol. 54, Issue 3
Fourier transform infrared spectrum of CH2NH: The ν1 band
journal, July 1985
- Halonen, Lauri; Duxbury, Geoffrey
- Chemical Physics Letters, Vol. 118, Issue 3
In pursuit of the ab initio limit for conformational energy prototypes
journal, June 1998
- Császár, Attila G.; Allen, Wesley D.; Schaefer, Henry F.
- The Journal of Chemical Physics, Vol. 108, Issue 23
Examining the ground and first excited states of methyl peroxy radical with high-level coupled-cluster theory
journal, July 2015
- Copan, Andreas V.; Schaefer, Henry F.; Agarwal, Jay
- Molecular Physics, Vol. 113, Issue 19-20
Relativistic Internally Contracted Multireference Electron Correlation Methods
journal, September 2015
- Shiozaki, Toru; Mizukami, Wataru
- Journal of Chemical Theory and Computation, Vol. 11, Issue 10
Spectroscopic effects of conical intersections of molecular potential energy surfaces
journal, July 1981
- Domcke, W.; Köppel, H.; Cederbaum, L. S.
- Molecular Physics, Vol. 43, Issue 4
Accurate vibrational spectra and magnetic properties of organic free radicals: The case of H2CN
journal, June 2005
- Barone, Vincenzo; Carbonniere, Philippe; Pouchan, Claude
- The Journal of Chemical Physics, Vol. 122, Issue 22
Infrared band intensities of formaldehyde and formaldehyde‐ d 2
journal, April 1982
- Nakanaga, Taisuke; Kondo, Shigeo; Saëki, Shinnosuke
- The Journal of Chemical Physics, Vol. 76, Issue 8
The adiabatic correction to molecular potential surfaces in the SCF approximation
journal, January 1984
- Sellers, Harrell; Pulay, Peter
- Chemical Physics Letters, Vol. 103, Issue 6
Multiconfiguration Self-Consistent Field and Multireference Configuration Interaction Methods and Applications
journal, December 2011
- Szalay, Péter G.; Müller, Thomas; Gidofalvi, Gergely
- Chemical Reviews, Vol. 112, Issue 1
The Raman Spectrum of Ethylene
journal, August 1956
- Feldman, T.; Romanko, J.; Welsh, H. L.
- Canadian Journal of Physics, Vol. 34, Issue 8
A systematic study of molecular vibrational anharmonicity and vibration—rotation interaction by self-consistent-field higher-derivative methods. Asymmetric top molecules
journal, July 1988
- Clabo, D. Allen; Allen, Wesley D.; Remington, Richard B.
- Chemical Physics, Vol. 123, Issue 2
Higher excitations in coupled-cluster theory
journal, August 2001
- Kállay, Mihály; Surján, Péter R.
- The Journal of Chemical Physics, Vol. 115, Issue 7
Experimental and Theoretical Study of the Electronic Spectrum of the Methylene Amidogen Radical (H 2 CN): Verification of the 2 A 1 ← 2 B 2 Assignment
journal, June 2006
- Teslja, Alexey; Dagdigian, Paul J.; Banck, Michael
- The Journal of Physical Chemistry A, Vol. 110, Issue 25
Gaussian basis sets for use in correlated molecular calculations. I. The atoms boron through neon and hydrogen
journal, January 1989
- Dunning, Thom H.
- The Journal of Chemical Physics, Vol. 90, Issue 2
The use of systematic sequences of wave functions for estimating the complete basis set, full configuration interaction limit in water
journal, May 1993
- Feller, David
- The Journal of Chemical Physics, Vol. 98, Issue 9
An efficient internally contracted multiconfiguration–reference configuration interaction method
journal, November 1988
- Werner, Hans‐Joachim; Knowles, Peter J.
- The Journal of Chemical Physics, Vol. 89, Issue 9
The spectroscopy of molecular reaction intermediates trapped in the solid rare gases
journal, January 2002
- Jacox, Marilyn E.
- Chemical Society Reviews, Vol. 31, Issue 2
Excited state dynamics of H2CN radicals
journal, November 1999
- Bernard, Eugene J.; Strazisar, Brian R.; Davis, H. Floyd
- Chemical Physics Letters, Vol. 313, Issue 3-4
Theoretical Investigation of the Structure and Vibrational Spectrum of the Electronic Ground State X̃( 1 A‘) of HSiCl
journal, May 2002
- Vázquez, J.; Stanton, John F.
- The Journal of Physical Chemistry A, Vol. 106, Issue 17
Approximate treatment of higher excitations in coupled-cluster theory
journal, December 2005
- Kállay, Mihály; Gauss, Jürgen
- The Journal of Chemical Physics, Vol. 123, Issue 21
A CASSCF and CASPT2 Study on the Excited States of s-trans -Formaldazine
journal, September 2008
- Luo, Cheng; Duan, Xue-mei; Liu, Jing-yao
- The Journal of Physical Chemistry A, Vol. 112, Issue 38
Parallelization Strategy for Large-Scale Vibronic Coupling Calculations
journal, October 2014
- Rabidoux, Scott M.; Eijkhout, Victor; Stanton, John F.
- The Journal of Physical Chemistry A, Vol. 118, Issue 51
Benchmark calculations for molecules in the gas phase: State-of-the-art coupled-cluster computations
journal, March 2010
- Puzzarini, Cristina; Barone, Vincenzo
- International Journal of Quantum Chemistry, Vol. 110, Issue 3
The microwave spectrum of the CH2N radical in the X̃ 2B2 ground electronic state
journal, March 1992
- Yamamoto, Satoshi; Saito, Shuji
- The Journal of Chemical Physics, Vol. 96, Issue 6
Photodissociation of formaldoxime and its methylated homologs: search for methylamidogen radical fluorescence
journal, August 1989
- Dagdigian, Paul J.; Anderson, William R.; Sausa, Rosario
- The Journal of Physical Chemistry, Vol. 93, Issue 16
Electron spin resonance study of the photolysis of formmaldazine
journal, January 1971
- Kamachi, Mikiharu; Kuwata, Keiji; Murahashi, Shunsuke
- The Journal of Physical Chemistry, Vol. 75, Issue 1
High resolution infrared spectrum of methyleneimine, CH 2 NH,in the 3 μm region
journal, September 1985
- Halonen, Lauri; Duxbury, Geoffrey
- The Journal of Chemical Physics, Vol. 83, Issue 5
Coupled-cluster methods including noniterative corrections for quadruple excitations
journal, August 2005
- Bomble, Yannick J.; Stanton, John F.; Kállay, Mihály
- The Journal of Chemical Physics, Vol. 123, Issue 5
The infrared spectrum of methylenimine
journal, June 1975
- Jacox, Marilyn E.; Milligan, Dolphus E.
- Journal of Molecular Spectroscopy, Vol. 56, Issue 3
Configuration interaction calculations on the nitrogen molecule
journal, January 1974
- Langhoff, Stephen R.; Davidson, Ernest R.
- International Journal of Quantum Chemistry, Vol. 8, Issue 1
Neutral rare-gas containing charge-transfer molecules in solid matrices. III. HXeCN, HXeNC, and HKrCN in Kr and Xe
journal, July 1998
- Pettersson, Mika; Lundell, Jan; Khriachtchev, Leonid
- The Journal of Chemical Physics, Vol. 109, Issue 2
Spectroscopic and Kinetic Investigation of Methylene Amidogen by Cavity Ring-Down Spectroscopy
journal, April 2003
- Nizamov, Boris; Dagdigian, Paul J.
- The Journal of Physical Chemistry A, Vol. 107, Issue 13
Predissociation of the Schumann-Runge bands of O2
journal, May 1975
- Julienne, Paul S.; Krauss, Morris
- Journal of Molecular Spectroscopy, Vol. 56, Issue 2
Spin-restricted open-shell coupled-cluster theory
journal, December 1997
- Szalay, Péter G.; Gauss, Jürgen
- The Journal of Chemical Physics, Vol. 107, Issue 21
The diagonal correction to the Born–Oppenheimer approximation: Its effect on the singlet–triplet splitting of CH 2 and other molecular effects
journal, April 1986
- Handy, Nicholas C.; Yamaguchi, Yukio; Schaefer, Henry F.
- The Journal of Chemical Physics, Vol. 84, Issue 8
Analysis of CH 2 a ̃ 1 A 1 (1,0,0) and (0,0,1) Coriolis‐coupled states, a ̃ 1 A 1 – X ̃ 3 B 1 spin–orbit coupling, and the equilibrium structure of CH 2 a ̃ 1 A 1 state
journal, December 1989
- Petek, Hrvoje; Nesbitt, David J.; Darwin, David C.
- The Journal of Chemical Physics, Vol. 91, Issue 11
ESR Detection of the Cyanogen and Methylene Imino Free Radicals
journal, April 1962
- Cochran, Edward L.; Adrian, Frank J.; Bowers, Vernon A.
- The Journal of Chemical Physics, Vol. 36, Issue 7
Perturbative corrections to account for triple excitations in closed and open shell coupled cluster theories
journal, September 1994
- Deegan, Miles J. O.; Knowles, Peter J.
- Chemical Physics Letters, Vol. 227, Issue 3
Carbon-13 hyperfine constants of methyleneamidogen, hydroxymethyleneamidogen and aminooxomethyl radicals
journal, June 1988
- McManus, Hugh J.; Fessenden, Richard W.; Chipman, Daniel M.
- The Journal of Physical Chemistry, Vol. 92, Issue 13
Application of systematic sequences of wave functions to the water dimer
journal, April 1992
- Feller, David
- The Journal of Chemical Physics, Vol. 96, Issue 8
New theoretical evidence for the nonlinearity of the triplet ground state of methylene
journal, August 1970
- Bender, Charles F.; Schaefer, Henry F.
- Journal of the American Chemical Society, Vol. 92, Issue 16
Electronic Absorption Spectra of Methanal Azine and the Methyleniminyl Free Radical
journal, March 1968
- Ogilvie, J. F.; Horne, D. G.
- The Journal of Chemical Physics, Vol. 48, Issue 5
The heat of formation of NCO
journal, September 1993
- East, Allan L. L.; Allen, Wesley D.
- The Journal of Chemical Physics, Vol. 99, Issue 6
Methylene: A Paradigm for Computational Quantum Chemistry
journal, March 1986
- Schaefer, H. F.
- Science, Vol. 231, Issue 4742
Approximate treatment of higher excitations in coupled-cluster theory. II. Extension to general single-determinant reference functions and improved approaches for the canonical Hartree–Fock case
journal, October 2008
- Kállay, Mihály; Gauss, Jürgen
- The Journal of Chemical Physics, Vol. 129, Issue 14
Cyanovinyl radical: an illustration of the poor performance of unrestricted perturbation theory and density functional theory procedures in calculating radical stabilization energies
journal, June 1999
- Parkinson, Christopher J.; Mayer, Paul M.; Radom, Leo
- Theoretical Chemistry Accounts: Theory, Computation, and Modeling (Theoretica Chimica Acta), Vol. 102, Issue 1-6
The puckering inversion barrier and vibrational spectrum of cyclopentene. A scaled quantum mechanical force field algorithm
journal, August 1992
- Allen, Wesley D.; Csaszar, Attila G.; Horner, David A.
- Journal of the American Chemical Society, Vol. 114, Issue 17
Pyrolysis of amines: Infrared spectrum of methyleneimine
journal, May 1984
- Hamada, Yoshiaki; Hashiguchi, Kazuko; Tsuboi, Masamichi
- Journal of Molecular Spectroscopy, Vol. 105, Issue 1
Detection of a new interstellar molecule, H2CN
journal, May 1994
- Ohishi, Masatoshi; McGonagle, Douglas; Irvine, William M.
- The Astrophysical Journal, Vol. 427
Chemical Reactions in Energetic Materials
journal, October 1992
- Adams, G. F.; Shaw, R. W.
- Annual Review of Physical Chemistry, Vol. 43, Issue 1
Vibrational and electronic spectra of the hydrogen atom + hydrogen cyanide reaction products trapped in solid argon
journal, December 1987
- Jacox, Marilyn E.
- The Journal of Physical Chemistry, Vol. 91, Issue 27
Measurement of the photoionization spectra and ionization thresholds of the methyleneamidogen and methyleneamidogen-d2 radicals
journal, October 1991
- Nesbitt, F. L.; Marston, G.; Stief, L. J.
- The Journal of Physical Chemistry, Vol. 95, Issue 20
A remarkable large effect of spin contamination on calculated vibrational frequencies
journal, June 1990
- Jensen, Frank
- Chemical Physics Letters, Vol. 169, Issue 6
The infrared spectrum of thioformaldehyde
journal, September 1971
- Johns, J. W. C.; Olson, W. B.
- Journal of Molecular Spectroscopy, Vol. 39, Issue 3
Electron-spin-resonance studies of HMX pyrolysis products
journal, January 1979
- Morgan, Cornelius U.; Beyer, Richard A.
- Combustion and Flame, Vol. 36
Infrared overtone spectroscopy and unimolecular decay dynamics of peroxynitrous acid
journal, March 2005
- Konen, Ian M.; Pollack, Ilana B.; Li, Eunice X. J.
- The Journal of Chemical Physics, Vol. 122, Issue 9
Basis-set convergence of correlated calculations on water
journal, June 1997
- Helgaker, Trygve; Klopper, Wim; Koch, Henrik
- The Journal of Chemical Physics, Vol. 106, Issue 23
Perturbative treatment of triple excitations in internally contracted multireference coupled cluster theory
journal, May 2012
- Hanauer, Matthias; Köhn, Andreas
- The Journal of Chemical Physics, Vol. 136, Issue 20
Theoretical investigation of ground and excited states of the methylene amidogene radical (H2CN)
journal, April 2004
- Eisfeld, Wolfgang
- The Journal of Chemical Physics, Vol. 120, Issue 13
Theoretical studies of the reactions of HCN with atomic hydrogen
journal, March 1985
- Bair, Raymond A.; Dunning, Thom. H.
- The Journal of Chemical Physics, Vol. 82, Issue 5
Dissociation Energy and Ionization Potential of Molecular Hydrogen
journal, November 1969
- Herzberg, G.
- Physical Review Letters, Vol. 23, Issue 19
Formation of nitrogenated organic aerosols in the Titan upper atmosphere
journal, July 2010
- Imanaka, Hiroshi; Smith, Mark A.
- Proceedings of the National Academy of Sciences, Vol. 107, Issue 28
Coupled-cluster characterization of the ground and excited states of the CH2N and CH2P radicals
journal, February 2001
- Brinkmann, Nicole R.; Wesolowski, Steven S.; Schaefer, Henry F.
- The Journal of Chemical Physics, Vol. 114, Issue 7
Photoelectron spectroscopy of CH 2 N −
journal, March 1991
- Cowles, Daniel C.; Travers, Michael J.; Frueh, Jennifer L.
- The Journal of Chemical Physics, Vol. 94, Issue 5
An efficient linear-scaling CCSD(T) method based on local natural orbitals
journal, September 2013
- Rolik, Zoltán; Szegedy, Lóránt; Ladjánszki, István
- The Journal of Chemical Physics, Vol. 139, Issue 9
Toward subchemical accuracy in computational thermochemistry: Focal point analysis of the heat of formation of NCO and [H,N,C,O] isomers
journal, June 2004
- Schuurman, Michael S.; Muir, Steven R.; Allen, Wesley D.
- The Journal of Chemical Physics, Vol. 120, Issue 24
Examining the ground and first excited states of methyl peroxy radical with high-level coupled-cluster theory
text, January 2015
- Copan, Andreas V.; Schaefer, Henry F.; Agarwal, Jay
- Taylor & Francis
Examining the ground and first excited states of methyl peroxy radical with high-level coupled-cluster theory
text, January 2015
- Copan, Andreas V.; Schaefer, Henry F.; Agarwal, Jay
- Taylor & Francis
Examining the ground and first excited states of methyl peroxy radical with high-level coupled-cluster theory
text, January 2015
- Copan, Andreas V.; Schaefer, Henry F.; Agarwal, Jay
- Taylor & Francis
Relativistic Internally Contracted Multireference Electron Correlation Methods
text, January 2015
- Shiozaki, Toru; Mizukami, Wataru
- arXiv
Works referencing / citing this record:
Reinterpretation of the electronic absorption spectrum of the methylene amidogen radical (H 2 CN)
journal, September 2018
- Abbott, Adam S.; Glick, Zach L.; Schaefer, Henry F.
- The Journal of Chemical Physics, Vol. 149, Issue 9
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal