COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin
Abstract
Cisplatin-based therapy is highly toxic, but moderately effective in most cancers. Concurrent inhibition of cyclooxygenase-2 (COX-2) and soluble epoxide hydrolase (sEH) results in antitumor activity and has organ-protective effects. The goal of this paper was to determine the antitumor activity of PTUPB, an orally bioavailable COX-2/sEH dual inhibitor, in combination with cisplatin and gemcitabine (GC) therapy. NSG mice bearing bladder cancer patient-derived xenografts were treated with vehicle, PTUPB, cisplatin, GC, or combinations thereof. Mouse experiments were performed with two different PDX models. PTUPB potentiated cisplatin and GC therapy, resulting in significantly reduced tumor growth and prolonged survival. PTUPB plus cisplatin was no more toxic than cisplatin single-agent treatment as assessed by body weight, histochemical staining of major organs, blood counts, and chemistry. The combination of PTUPB and cisplatin increased apoptosis and decreased phosphorylation in the MAPK/ERK and PI3K/AKT/mTOR pathways compared with controls. PTUPB treatment did not alter platinum–DNA adduct levels, which is the most critical step in platinum-induced cell death. The in vitro study using the combination index method showed modest synergy between PTUPB and platinum agents only in 5637 cell line among several cell lines examined. However, PTUPB is very active in vivo by inhibiting angiogenesis. Finally, PTUPB potentiatedmore »
- Authors:
-
- Univ. of California Davis, Sacramento, CA (United States). Dept. of Internal Medicine. School of Medicine; Fourth Military Medical Univ., Xi'an (China). Dept. of Urology. Xijing Hospital
- Univ. of California Davis, Sacramento, CA (United States). Dept. of Internal Medicine. School of Medicine
- Univ. of California Davis, Sacramento, CA (United States). Dept. of Biochemistry and Molecular Medicine. School of Medicine
- Univ. of California Davis, Sacramento, CA (United States). Dept. of Internal Medicine. School of Medicine; Wuhan Univ. (China). Dept. of Urology. Renmin Hospital
- Univ. of California, Davis, CA (United States). Dept. of Entomology and Nematology
- Lawrence Livermore National Lab. (LLNL), Livermore, CA (United States)
- The Jackson Lab., Bar Harbor, ME (United States)
- Fourth Military Medical Univ., Xi'an (China). Dept. of Urology. Xijing Hospital
- Univ. of California Davis, Sacramento, CA (United States). Dept. of Urology. School of Medicine. Comprehensive Cancer Center
- Univ. of California Davis, Sacramento, CA (United States). Dept. of Internal Medicine. Dept. of Urology. School of Medicine. Comprehensive Cancer Center; VA Northern California Health Care System, Rancho Cordova, CA (United States)
- Publication Date:
- Research Org.:
- Lawrence Livermore National Lab. (LLNL), Livermore, CA (United States); Univ. of California Davis, Sacramento, CA (United States)
- Sponsoring Org.:
- USDOE; Dept. of Veterans Affairs (VA) (United States); National Inst. of Health (NIH) (United States)
- OSTI Identifier:
- 1438685
- Report Number(s):
- LLNL-JRNL-741222
Journal ID: ISSN 1535-7163
- Grant/Contract Number:
- AC52-07NA27344; I01 BX001784; 2 P30 CA 0933730; R01 ES002710; P42 ES04699; HHSN261201200048C; R01 DK103616; U54 NS079202
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Molecular Cancer Therapeutics
- Additional Journal Information:
- Journal Volume: 17; Journal Issue: 2; Journal ID: ISSN 1535-7163
- Publisher:
- American Association for Cancer Research (AACR)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 60 APPLIED LIFE SCIENCES
Citation Formats
Wang, Fuli, Zhang, Hongyong, Ma, Ai-Hong, Yu, Weimin, Zimmermann, Maike, Yang, Jun, Hwang, Sung Hee, Zhu, Daniel, Lin, Tzu-yin, Malfatti, Michael, Turteltaub, Kenneth W., Henderson, Paul T., Airhart, Susan, Hammock, Bruce D., Yuan, Jianlin, de Vere White, Ralph W., and Pan, Chong-Xian. COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin. United States: N. p., 2017.
Web. doi:10.1158/1535-7163.MCT-16-0818.
Wang, Fuli, Zhang, Hongyong, Ma, Ai-Hong, Yu, Weimin, Zimmermann, Maike, Yang, Jun, Hwang, Sung Hee, Zhu, Daniel, Lin, Tzu-yin, Malfatti, Michael, Turteltaub, Kenneth W., Henderson, Paul T., Airhart, Susan, Hammock, Bruce D., Yuan, Jianlin, de Vere White, Ralph W., & Pan, Chong-Xian. COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin. United States. https://doi.org/10.1158/1535-7163.MCT-16-0818
Wang, Fuli, Zhang, Hongyong, Ma, Ai-Hong, Yu, Weimin, Zimmermann, Maike, Yang, Jun, Hwang, Sung Hee, Zhu, Daniel, Lin, Tzu-yin, Malfatti, Michael, Turteltaub, Kenneth W., Henderson, Paul T., Airhart, Susan, Hammock, Bruce D., Yuan, Jianlin, de Vere White, Ralph W., and Pan, Chong-Xian. Thu .
"COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin". United States. https://doi.org/10.1158/1535-7163.MCT-16-0818. https://www.osti.gov/servlets/purl/1438685.
@article{osti_1438685,
title = {COX-2/sEH Dual Inhibitor PTUPB Potentiates the Antitumor Efficacy of Cisplatin},
author = {Wang, Fuli and Zhang, Hongyong and Ma, Ai-Hong and Yu, Weimin and Zimmermann, Maike and Yang, Jun and Hwang, Sung Hee and Zhu, Daniel and Lin, Tzu-yin and Malfatti, Michael and Turteltaub, Kenneth W. and Henderson, Paul T. and Airhart, Susan and Hammock, Bruce D. and Yuan, Jianlin and de Vere White, Ralph W. and Pan, Chong-Xian},
abstractNote = {Cisplatin-based therapy is highly toxic, but moderately effective in most cancers. Concurrent inhibition of cyclooxygenase-2 (COX-2) and soluble epoxide hydrolase (sEH) results in antitumor activity and has organ-protective effects. The goal of this paper was to determine the antitumor activity of PTUPB, an orally bioavailable COX-2/sEH dual inhibitor, in combination with cisplatin and gemcitabine (GC) therapy. NSG mice bearing bladder cancer patient-derived xenografts were treated with vehicle, PTUPB, cisplatin, GC, or combinations thereof. Mouse experiments were performed with two different PDX models. PTUPB potentiated cisplatin and GC therapy, resulting in significantly reduced tumor growth and prolonged survival. PTUPB plus cisplatin was no more toxic than cisplatin single-agent treatment as assessed by body weight, histochemical staining of major organs, blood counts, and chemistry. The combination of PTUPB and cisplatin increased apoptosis and decreased phosphorylation in the MAPK/ERK and PI3K/AKT/mTOR pathways compared with controls. PTUPB treatment did not alter platinum–DNA adduct levels, which is the most critical step in platinum-induced cell death. The in vitro study using the combination index method showed modest synergy between PTUPB and platinum agents only in 5637 cell line among several cell lines examined. However, PTUPB is very active in vivo by inhibiting angiogenesis. Finally, PTUPB potentiated the antitumor activity of cisplatin-based treatment without increasing toxicity in vivo and has potential for further development as a combination chemotherapy partner.},
doi = {10.1158/1535-7163.MCT-16-0818},
journal = {Molecular Cancer Therapeutics},
number = 2,
volume = 17,
place = {United States},
year = {Thu Dec 28 00:00:00 EST 2017},
month = {Thu Dec 28 00:00:00 EST 2017}
}
Web of Science
Figures / Tables:
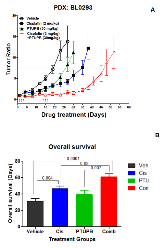 Figure 1:: Potentiation of cisplatin anti-tumor activity by PTUPB. A). Tumor growth in the NSG-PDX bladder cancer mouse model. When the volume of the tumor xenografts reached approximately 0.1~0.2 cm3, mice were treated with PEG 400 control, single agent cisplatin (2mg/kg, i.v., Day 1, 2, 3, 14, 15, and 16,more »
Figure 1:: Potentiation of cisplatin anti-tumor activity by PTUPB. A). Tumor growth in the NSG-PDX bladder cancer mouse model. When the volume of the tumor xenografts reached approximately 0.1~0.2 cm3, mice were treated with PEG 400 control, single agent cisplatin (2mg/kg, i.v., Day 1, 2, 3, 14, 15, and 16,more »
Works referencing / citing this record:
Cyclooxygenase-2 in cancer: A review: HASHEMI et al.
journal, October 2018
- Hashemi Goradel, Nasser; Najafi, Masoud; Salehi, Eniseh
- Journal of Cellular Physiology, Vol. 234, Issue 5
Suppression of chemotherapy-induced cytokine/lipid mediator surge and ovarian cancer by a dual COX-2/sEH inhibitor
journal, January 2019
- Gartung, Allison; Yang, Jun; Sukhatme, Vikas P.
- Proceedings of the National Academy of Sciences, Vol. 116, Issue 5
COX‐2/sEH dual inhibitor PTUPB alleviates bleomycin‐induced pulmonary fibrosis in mice via inhibiting senescence
journal, November 2019
- Zhang, Chen‐Yu; Duan, Jia‐Xi; Yang, Hui‐Hui
- The FEBS Journal, Vol. 287, Issue 8
Curbing Lipids: Impacts ON Cancer and Viral Infection
journal, February 2019
- Dutta, Anika; Sharma-Walia, Neelam
- International Journal of Molecular Sciences, Vol. 20, Issue 3
Radiocarbon Tracers in Toxicology and Medicine: Recent Advances in Technology and Science
journal, May 2019
- Malfatti, Michael A.; Buchholz, Bruce A.; Enright, Heather A.
- Toxics, Vol. 7, Issue 2
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal