Identification and Characterization of JAK2 Pseudokinase Domain Small Molecule Binders
Abstract
Janus kinases (JAKs) regulate hematopoiesis via the cytokine-mediated JAK-STAT signaling pathway. JAKs contain tandem C-terminal pseudokinase (JH2) and tyrosine kinase (JH1) domains. The JAK2 pseudokinase domain adopts a protein kinase fold and, despite its pseudokinase designation, binds ATP with micromolar affinity. Recent evidence shows that displacing ATP from the JAK2 JH2 domain alters the hyperactivation state of the oncogenic JAK2 V617F protein while sparing the wild type JAK2 protein. In this study, small molecule binders of JAK2 JH2 were identified via an in vitro screen. Top hits were characterized using biophysical and structural approaches. Development of pseudokinase-selective compounds may offer novel pharmacological opportunities for treating cancers driven by JAK2 V617F and other oncogenic JAK mutants.
- Authors:
-
- Yale Univ., New Haven, CT (United States)
- Tampere Univ. of Technology (Finland)
- Publication Date:
- Research Org.:
- Argonne National Laboratory (ANL), Argonne, IL (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1390880
- Grant/Contract Number:
- 1S10OD018007
- Resource Type:
- Accepted Manuscript
- Journal Name:
- ACS Medicinal Chemistry Letters
- Additional Journal Information:
- Journal Volume: 8; Journal Issue: 6; Journal ID: ISSN 1948-5875
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- ENGLISH
- Subject:
- 59 BASIC BIOLOGICAL SCIENCES; JAK2; Pseudokinase; JH2; MPN; Small molecule
Citation Formats
Puleo, David E., Kucera, Kaury, Hammarén, Henrik M., Ungureanu, Daniela, Newton, Ana S., Silvennoinen, Olli, Jorgensen, William L., and Schlessinger, Joseph. Identification and Characterization of JAK2 Pseudokinase Domain Small Molecule Binders. United States: N. p., 2017.
Web. doi:10.1021/acsmedchemlett.7b00153.
Puleo, David E., Kucera, Kaury, Hammarén, Henrik M., Ungureanu, Daniela, Newton, Ana S., Silvennoinen, Olli, Jorgensen, William L., & Schlessinger, Joseph. Identification and Characterization of JAK2 Pseudokinase Domain Small Molecule Binders. United States. https://doi.org/10.1021/acsmedchemlett.7b00153
Puleo, David E., Kucera, Kaury, Hammarén, Henrik M., Ungureanu, Daniela, Newton, Ana S., Silvennoinen, Olli, Jorgensen, William L., and Schlessinger, Joseph. Mon .
"Identification and Characterization of JAK2 Pseudokinase Domain Small Molecule Binders". United States. https://doi.org/10.1021/acsmedchemlett.7b00153. https://www.osti.gov/servlets/purl/1390880.
@article{osti_1390880,
title = {Identification and Characterization of JAK2 Pseudokinase Domain Small Molecule Binders},
author = {Puleo, David E. and Kucera, Kaury and Hammarén, Henrik M. and Ungureanu, Daniela and Newton, Ana S. and Silvennoinen, Olli and Jorgensen, William L. and Schlessinger, Joseph},
abstractNote = {Janus kinases (JAKs) regulate hematopoiesis via the cytokine-mediated JAK-STAT signaling pathway. JAKs contain tandem C-terminal pseudokinase (JH2) and tyrosine kinase (JH1) domains. The JAK2 pseudokinase domain adopts a protein kinase fold and, despite its pseudokinase designation, binds ATP with micromolar affinity. Recent evidence shows that displacing ATP from the JAK2 JH2 domain alters the hyperactivation state of the oncogenic JAK2 V617F protein while sparing the wild type JAK2 protein. In this study, small molecule binders of JAK2 JH2 were identified via an in vitro screen. Top hits were characterized using biophysical and structural approaches. Development of pseudokinase-selective compounds may offer novel pharmacological opportunities for treating cancers driven by JAK2 V617F and other oncogenic JAK mutants.},
doi = {10.1021/acsmedchemlett.7b00153},
journal = {ACS Medicinal Chemistry Letters},
number = 6,
volume = 8,
place = {United States},
year = {Mon May 22 00:00:00 EDT 2017},
month = {Mon May 22 00:00:00 EDT 2017}
}
Web of Science
Figures / Tables:
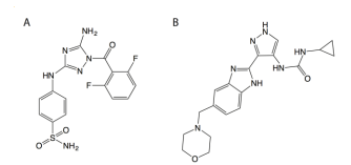 Figure 1: Chemical structures of the two top hits from the fluorescence polarization screen, (A) JNJ-7706621 and (B) AT9283.
Figure 1: Chemical structures of the two top hits from the fluorescence polarization screen, (A) JNJ-7706621 and (B) AT9283.
Works referenced in this record:
The JAK-STAT Pathway: Impact on Human Disease and Therapeutic Intervention
journal, January 2015
- O'Shea, John J.; Schwartz, Daniella M.; Villarino, Alejandro V.
- Annual Review of Medicine, Vol. 66, Issue 1
Regulation of the Jak2 Tyrosine Kinase by Its Pseudokinase Domain
journal, May 2000
- Saharinen, Pipsa; Takaluoma, Kati; Silvennoinen, Olli
- Molecular and Cellular Biology, Vol. 20, Issue 10
Structure of the pseudokinase-kinase domains from protein kinase TYK2 reveals a mechanism for Janus kinase (JAK) autoinhibition
journal, May 2014
- Lupardus, P. J.; Ultsch, M.; Wallweber, H.
- Proceedings of the National Academy of Sciences, Vol. 111, Issue 22
Molecular basis for pseudokinase-dependent autoinhibition of JAK2 tyrosine kinase
journal, June 2014
- Shan, Yibing; Gnanasambandan, Kavitha; Ungureanu, Daniela
- Nature Structural & Molecular Biology, Vol. 21, Issue 7
Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders
journal, March 2005
- Baxter, E.; Scott, L.; Campbell, P.
- The Lancet, Vol. 365, Issue 9464
A unique clonal JAK2 mutation leading to constitutive signalling causes polycythaemia vera
journal, March 2005
- James, Chloé; Ugo, Valérie; Le Couédic, Jean-Pierre
- Nature, Vol. 434, Issue 7037
A Gain-of-Function Mutation of JAK2 in Myeloproliferative Disorders
journal, April 2005
- Kralovics, Robert; Passamonti, Francesco; Buser, Andreas S.
- New England Journal of Medicine, Vol. 352, Issue 17
Activating mutation in the tyrosine kinase JAK2 in polycythemia vera, essential thrombocythemia, and myeloid metaplasia with myelofibrosis
journal, April 2005
- Levine, Ross L.; Wadleigh, Martha; Cools, Jan
- Cancer Cell, Vol. 7, Issue 4
Structure of a pseudokinase-domain switch that controls oncogenic activation of Jak kinases
journal, September 2013
- Toms, Angela V.; Deshpande, Anagha; McNally, Randall
- Nature Structural & Molecular Biology, Vol. 20, Issue 10
The pseudokinase domain of JAK2 is a dual-specificity protein kinase that negatively regulates cytokine signaling
journal, August 2011
- Ungureanu, Daniela; Wu, Jinhua; Pekkala, Tuija
- Nature Structural & Molecular Biology, Vol. 18, Issue 9
Crystal structures of the JAK2 pseudokinase domain and the pathogenic mutant V617F
journal, July 2012
- Bandaranayake, Rajintha M.; Ungureanu, Daniela; Shan, Yibing
- Nature Structural & Molecular Biology, Vol. 19, Issue 8
Structural and Functional Characterization of the JH2 Pseudokinase Domain of JAK Family Tyrosine Kinase 2 (TYK2)
journal, November 2015
- Min, Xiaoshan; Ungureanu, Daniela; Maxwell, Sarah
- Journal of Biological Chemistry, Vol. 290, Issue 45
ATP binding to the pseudokinase domain of JAK2 is critical for pathogenic activation
journal, March 2015
- Hammarén, Henrik M.; Ungureanu, Daniela; Grisouard, Jean
- Proceedings of the National Academy of Sciences, Vol. 112, Issue 15
1-Acyl-1 H -[1,2,4]triazole-3,5-diamine Analogues as Novel and Potent Anticancer Cyclin-Dependent Kinase Inhibitors: Synthesis and Evaluation of Biological Activities
journal, June 2005
- Lin, Ronghui; Connolly, Peter J.; Huang, Shenlin
- Journal of Medicinal Chemistry, Vol. 48, Issue 13
A quantitative analysis of kinase inhibitor selectivity
journal, January 2008
- Karaman, Mazen W.; Herrgard, Sanna; Treiber, Daniel K.
- Nature Biotechnology, Vol. 26, Issue 1
Fragment-Based Discovery of the Pyrazol-4-yl Urea (AT9283), a Multitargeted Kinase Inhibitor with Potent Aurora Kinase Activity †
journal, January 2009
- Howard, Steven; Berdini, Valerio; Boulstridge, John A.
- Journal of Medicinal Chemistry, Vol. 52, Issue 2
JAK2 V617F Constitutive Activation Requires JH2 Residue F595: A Pseudokinase Domain Target for Specific Inhibitors
journal, June 2010
- Dusa, Alexandra; Mouton, Céline; Pecquet, Christian
- PLoS ONE, Vol. 5, Issue 6
Structure of the pseudokinase-kinase domains from protein kinase TYK2 reveals a mechanism for Janus kinase (JAK) autoinhibition
journal, May 2014
- Lupardus, P. J.; Ultsch, M.; Wallweber, H.
- Proceedings of the National Academy of Sciences, Vol. 111, Issue 22
Autoinhibition of Jak2 Tyrosine Kinase Is Dependent on Specific Regions in Its Pseudokinase Domain
journal, April 2003
- Saharinen, Pipsa; Vihinen, Mauno; Silvennoinen, Olli
- Molecular Biology of the Cell, Vol. 14, Issue 4
Regulation of the Jak2 Tyrosine Kinase by Its Pseudokinase Domain
journal, January 2000
- Saharinen, Pipsa; Takaluoma, Kati; Silvennoinen, Olli
- Molecular and Cellular Biology, Vol. 20, Issue 10
Works referencing / citing this record:
Covalent inhibitors of EGFR family protein kinases induce degradation of human Tribbles 2 (TRIB2) pseudokinase in cancer cells
journal, September 2018
- Foulkes, Daniel M.; Byrne, Dominic P.; Yeung, Wayland
- Science Signaling, Vol. 11, Issue 549
Prospects for pharmacological targeting of pseudokinases
journal, March 2019
- Kung, Jennifer E.; Jura, Natalia
- Nature Reviews Drug Discovery
Janus kinases to jakinibs: from basic insights to clinical practice
journal, February 2019
- Gadina, Massimo; Le, Mimi T.; Schwartz, Daniella M.
- Rheumatology, Vol. 58, Issue Supplement_1
The PEAK family of pseudokinases, their role in cell signalling and cancer
journal, November 2019
- Patel, Onisha; Roy, Michael J.; Murphy, James M.
- The FEBS Journal, Vol. 287, Issue 19
JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders
journal, January 2018
- Vainchenker, William; Leroy, Emilie; Gilles, Laure
- F1000Research, Vol. 7
Repurposing covalent EGFR/HER2 inhibitors for on-target degradation of human Tribbles 2 (TRIB2) pseudokinase
posted_content, April 2018
- Foulkes, Daniel M.; Byrne, Dominic P.; Bailey, Fiona P.
JAK inhibitors for the treatment of myeloproliferative neoplasms and other disorders
journal, January 2018
- Vainchenker, William; Leroy, Emilie; Gilles, Laure
- F1000Research, Vol. 7
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal