Solution Structure of an Intramembrane Aspartyl Protease via Small Angle Neutron Scattering
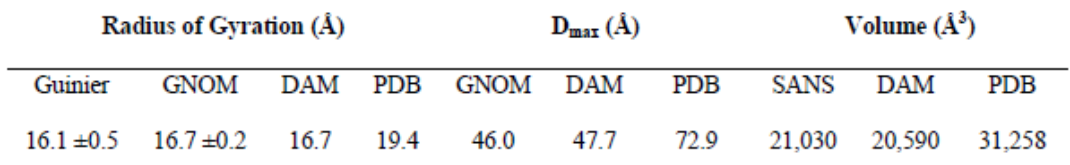
Intramembrane aspartyl proteases (IAPs) comprise one of four families of integral membrane proteases that hydrolyze substrates within the hydrophobic lipid bilayer. IAPs include signal peptide peptidase, which processes remnant signal peptides from nascent polypeptides in the endoplasmic reticulum, and presenilin, the catalytic component of the γ-secretase complex that processes Notch and amyloid precursor protein. Despite their broad biomedical reach, basic structure-function relationships of IAPs remain active areas of research. Characterization of membrane-bound proteins is notoriously challenging due to their inherently hydrophobic character. For IAPs, oligomerization state in solution is one outstanding question, with previous proposals for monomer, dimer, tetramer, and octamer. Here we used small angle neutron scattering (SANS) to characterize n-dodecyl-β-D-maltopyranoside (DDM) detergent solutions containing and absent a microbial IAP ortholog. A unique feature of SANS is the ability to modulate the solvent composition to mask all but the enzyme of interest. The signal from the IAP was enhanced by deuteration and, uniquely, scattering from DDM and buffers were matched by the use of both tail-deuterated DDM and D2O. The radius of gyration calculated for IAP and the corresponding ab initio consensus model are consistent with a monomer. The model is slightly smaller than the crystallographic IAP monomer, suggesting a more compact protein in solution compared with the crystal lattice. In conclusion, our study provides direct insight into the oligomeric state of purified IAP in surfactant solution, and demonstrates the utility of fully contrast-matching the detergent in SANS to characterize other intramembrane proteases and their membrane-bound substrates.
- Research Organization:
- Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Biological and Environmental Research (BER); USDOE Office of Science (SC), Basic Energy Sciences (BES); National Science Foundation (NSF)
- Grant/Contract Number:
- AC05-00OR22725; DMR-0520547; 0845445
- OSTI ID:
- 1691827
- Alternate ID(s):
- OSTI ID: 1422538
- Journal Information:
- Biophysical Journal, Journal Name: Biophysical Journal Vol. 114 Journal Issue: 3; ISSN 0006-3495
- Publisher:
- ElsevierCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Characterization of the γ-secretase subunit interactome in Arabidopsis thaliana
|
journal | January 2019 |
Neutron scattering in the biological sciences: progress and prospects
|
journal | December 2018 |
Similar Records
Crystal Structure of a Rhomboid Family Intramembrane Protease.
Crystal structure of a self-assembling lipopeptide detergent at 1.20 Å