Reply to: “Pitfalls in identifying active catalyst species”
- Washington State Univ., Pullman, WA (United States)
- Univ. of New Mexico, Albuquerque, NM (United States)
- Eindhoven Univ. of Technology (Netherlands)
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States). Environmental Molecular Sciences Lab. (EMSL)
- Washington State Univ., Pullman, WA (United States); Pacific Northwest National Lab. (PNNL), Richland, WA (United States). Inst. for Integrated Catalysis
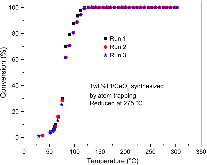
In Pereira-Hernández et al., we reported the influence of the high-temperature vapor-phase synthesis method (also called atom trapping, or AT) on the activity for CO oxidation of a Pt/CeO2 catalyst, compared to a conventional synthesis method (strong electrostatic adsorption, or SEA). The findings suggest that the AT method leads to increased activity compared to the SEA method, and this is related to improved redox properties of the support at low temperature. Recently, Ren and Chen questioned the interpretation of the results and suggested alternative explanations for the findings. However, as addressed in this paper, we are firmly of the opinion that the original analysis, results, and conclusions provided in Pereira-Hernández et al. are valid and accurately explain the phenomena observed.
- Research Organization:
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States). Environmental Molecular Sciences Lab. (EMSL)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES). Chemical Sciences, Geosciences & Biosciences Division; USDOE Office of Energy Efficiency and Renewable Energy (EERE), Transportation Office. Vehicle Technologies Office; Dutch Ministry of Education, Culture and Science (OCW); The Dutch Research Council (NWO); USDOE Office of Science (SC), Biological and Environmental Research (BER)
- Grant/Contract Number:
- AC05-76RL01830; FG02-05ER15712
- OSTI ID:
- 1663195
- Report Number(s):
- PNNL-SA-156008
- Journal Information:
- Nature Communications, Vol. 11, Issue 1; ISSN 2041-1723
- Publisher:
- Nature Publishing GroupCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Similar Records
Environmentally benign synthesis of a PGM-free catalyst for low temperature CO oxidation
Designing ultrastable Pt/CeO2-Al2O3 nanosheet catalysts for three-way catalysts applications