Electrochemical Impedance Spectroscopy and Cyclic Voltammetry Methods for Monitoring SmCl3 Concentration in Molten Eutectic LiCl-KCl
- Idaho National Lab. (INL), Idaho Falls, ID (United States)
- Boise State Univ., ID (United States)
- Univ. of Utah, Salt Lake City, UT (United States)
- Virginia Commonwealth Univ., Richmond, VA (United States)
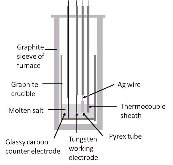
Molten salt solutions consisting of eutectic LiCl-KCl and concentrations of samarium chloride (0.5 to 3.0 wt%) at 500 C were analyzed using both cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS). The CV technique gave the average diffusion coefficient for Sm3+ over the concentration range. Equipped with Sm3+ diffusion coefficient, the Randles-Sevcik equation predicted Sm3+ concentration values that agree with the given experimental values. From CV measurements; the anodic, cathodic, and half-peak potentials were identified and subsequently used as a parameter to acquire EIS spectra. A six-element Voigt model was used to model the EIS data in terms of resistance-time constant pairs. The lowest resistances were observed at the half-peak potential with the associated resistance-time constant pairs characterizing the reversible reaction between Sm3+ and Sm2+. By extrapolation, the Voigt model estimated the polarization resistance and established a polarization resistance-concentration relationship.
- Research Organization:
- Idaho National Lab. (INL), Idaho Falls, ID (United States)
- Sponsoring Organization:
- USDOE Laboratory Directed Research and Development (LDRD) Program; USDOE Office of Nuclear Energy (NE)
- Grant/Contract Number:
- AC07-05ID14517
- OSTI ID:
- 1631811
- Report Number(s):
- INL/JOU-15-34095-Rev000; TRN: US2201259
- Journal Information:
- Journal of Nuclear Fuel Cycle and Waste Technology, Vol. 18, Issue 1; ISSN 1738-1894
- Publisher:
- Korean Radioactive Waste SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Similar Records
Electrochemical and Thermodynamic Properties of U in LiCl-KCl-UCl{sub 3} salt system
Determination and Comparison of Parameters from Cyclic Voltammetry of LaCl3 in Eutectic LiCl-KCl