Substituent effects on energetics and crystal morphology modulate singlet fission in 9,10-bis(phenylethynyl)anthracenes
Journal Article
·
· Journal of Chemical Physics
- Northwestern Univ., Evanston, IL (United States); DOE/OSTI
- Northwestern Univ., Evanston, IL (United States)
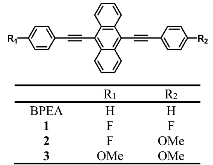
Singlet fission (SF) converts a singlet exciton into two triplet excitons in two or more electronically coupled organic chromophores, which may then be used to increase solar cell efficiency. Many known SF chromophores are unsuitable for device applications due to chemical instability or low triplet state energies. The results described here show that efficient SF occurs in derivatives of 9,10-bis(phenylethynyl)anthracene (BPEA), which is a highly robust and tunable chromophore. Fluoro and methoxy substituents at the 4- and 4'-positions of the BPEA phenyl groups control the intermolecular packing in the crystal structure, which alters the interchromophore electronic coupling, while also changing the SF energetics. The lowest excited singlet state (S1) energy of 4,4'-difluoro-BPEA is higher than that of BPEA so that the increased thermodynamic favorability of SF results in a (16 ± 2 ps)-1 SF rate and a 180% ± 16% triplet yield, which is about an order of magnitude faster than BPEA with a comparable triplet yield. By contrast, 4-fluoro-4'-methoxy-BPEA and 4,4'-dimethoxy-BPEA have slower SF rates, (90 ± 20 ps)-1 and (120 ± 10 ps)-1, and lower triplet yields, (110 ± 4)% and (168 ± 7)%, respectively, than 4,4'-difluoro-BPEA. These differences are attributed to changes in the crystal structure controlling interchromophore electronic coupling as well as SF energetics in these polycrystalline solids.
- Research Organization:
- Energy Frontier Research Centers (EFRC) (United States). Center for Light Energy Activated Redox Processes (LEAP); Northwestern Univ., Evanston, IL (United States)
- Sponsoring Organization:
- National Science Foundation (NSF); US Air Force Office of Scientific Research (AFOSR); USDOE; USDOE Office of Science (SC), Basic Energy Sciences (BES) (SC-22)
- Grant/Contract Number:
- FG02-99ER14999; SC0001059
- OSTI ID:
- 1610423
- Alternate ID(s):
- OSTI ID: 1544909
- Journal Information:
- Journal of Chemical Physics, Journal Name: Journal of Chemical Physics Journal Issue: 4 Vol. 151; ISSN 0021-9606
- Publisher:
- American Institute of Physics (AIP)Copyright Statement
- Country of Publication:
- United States
- Language:
- English
Similar Records
Singlet Fission via an Excimer-Like Intermediate in 3,6-Bis(thiophen-2-yl)diketopyrrolopyrrole Derivatives
Singlet Exciton Fission in Thin Films of tert -Butyl-Substituted Terrylenes
Emerging Design Principles for Enhanced Solar Energy Utilization with Singlet Fission
Journal Article
·
Sun Aug 21 20:00:00 EDT 2016
· Journal of the American Chemical Society
·
OSTI ID:1388173
Singlet Exciton Fission in Thin Films of tert -Butyl-Substituted Terrylenes
Journal Article
·
Thu May 07 00:00:00 EDT 2015
· Journal of Physical Chemistry. A, Molecules, Spectroscopy, Kinetics, Environment, and General Theory
·
OSTI ID:1392055
Emerging Design Principles for Enhanced Solar Energy Utilization with Singlet Fission
Journal Article
·
Mon Jan 28 19:00:00 EST 2019
· Journal of Physical Chemistry. C
·
OSTI ID:1496846