High-Level VSCF/VCI Calculations Decode the Vibrational Spectrum of the Aqueous Proton
Journal Article
·
· Journal of Physical Chemistry. B, Condensed Matter, Materials, Surfaces, Interfaces and Biophysical Chemistry
- Emory Univ., Atlanta, GA (United States); The University of Chicago
- Univ. of Chicago, IL (United States). James Frank Inst., and Inst. for Biophysical Dynamics
- Emory Univ., Atlanta, GA (United States)
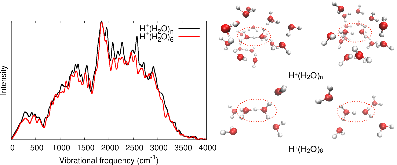
We report that the hydrated excess proton is a common species in aqueous chemistry which complexes with water in a variety of structures. The infrared spectrum of the aqueous proton is particularly sensitive to this array of structures, which manifests as continuous IR absorption from 1000-3000 cm-1 known as the “proton continuum”. Because of the extreme breadth of the continuum and strong anharmonicity of the involved vibrational modes, this spectrum has eluded straightforward interpretation and simulation. Using protonated water hexamer clusters from reactive molecular dynamics trajectories, and focusing on their central H+(H2O)2 structures’ spectral contribution, we reproduce the linear IR spectrum of the aqueous proton with a high-level local- monomer quantum method and highly accurate many-body potential energy surface. The accuracy of this approach is first verified in the vibrational spectra of the two isomers of the protonated water hexamer in the gas phase. We then apply this approach to 800 H+(H2O)6 clusters, also written as [H+(H2O)2](H2O)4, drawn from MS-EVB simulations of the bulk liquid to calculate the infrared spectrum of the aqueous proton complex. Incorporation of anharmonic effects to the vibrational potential and quantum mechanical treatment of the proton produce better agreement to the infrared spectrum compared to the double-harmonic approximation. We assess the correlation of the pro- ton stretching mode with different atomistic coordinates, finding the best correlation with $$\langle$$ROH$$\rangle$$, the expectation value of the proton-oxygen distance ROH. Finally, we also decompose the IR spectrum based on normal mode vibrations and $$\langle$$ROH$$\rangle$$ to provide insight on how different frequency regions in the continuum report on different configurations, vibrational modes, and mode couplings.
- Research Organization:
- Univ. of Chicago, IL (United States)
- Sponsoring Organization:
- National Science Foundation (NSF); USDOE Office of Science (SC), Basic Energy Sciences (BES) (SC-22)
- Grant/Contract Number:
- SC0014305
- OSTI ID:
- 1599995
- Journal Information:
- Journal of Physical Chemistry. B, Condensed Matter, Materials, Surfaces, Interfaces and Biophysical Chemistry, Journal Name: Journal of Physical Chemistry. B, Condensed Matter, Materials, Surfaces, Interfaces and Biophysical Chemistry Journal Issue: 33 Vol. 123; ISSN 1520-6106
- Publisher:
- American Chemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Nature of Excess Hydrated Proton at the Water–Air Interface
|
journal | December 2019 |
Similar Records
Decoding the 2D IR spectrum of the aqueous proton with high-level VSCF/VCI calculations
Anharmonic exciton dynamics and energy dissipation in liquid water from two-dimensional infrared spectroscopy
Broadband 2D IR spectroscopy reveals dominant asymmetric H5O2+ proton hydration structures in acid solutions
Journal Article
·
Tue Sep 22 20:00:00 EDT 2020
· Journal of Chemical Physics
·
OSTI ID:1761055
Anharmonic exciton dynamics and energy dissipation in liquid water from two-dimensional infrared spectroscopy
Journal Article
·
Wed Aug 31 20:00:00 EDT 2016
· Journal of Chemical Physics
·
OSTI ID:1600624
Broadband 2D IR spectroscopy reveals dominant asymmetric H5O2+ proton hydration structures in acid solutions
Journal Article
·
Sun Jul 29 20:00:00 EDT 2018
· Nature Chemistry
·
OSTI ID:1480907