Hard-Magnet L10-CoPt Nanoparticles Advance Fuel Cell Catalysis
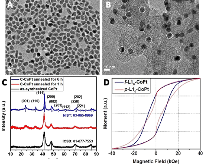
Stabilizing transition metals (M) in MPt alloy under acidic conditions is challenging, yet crucial to boost Pt catalysis toward oxygen reduction reaction (ORR). We synthesized ~9 nm hard-magnet core/shell L10-CoPt/Pt nanoparticles with 2–3 atomic layers of strained Pt shell for ORR. At 60°C in acid, the hard-magnet L10-CoPt better stabilizes Co (5% loss after 24 hr) than soft-magnet A1-CoPt (34% loss in 7 hr). L10-CoPt/Pt achieves mass activities (MA) of 0.56 A/mgPt initially and 0.45 A/mgPt after 30,000 voltage cycles in the membrane electrode assembly at 80°C, exceeding the DOE 2020 targets on Pt activity and durability (0.44 A/mgPt in MA and <40% loss in MA after 30,000 cycles). Lastly, density functional theory calculations suggest that the ligand effect of Co and the biaxial strain (-4.50%/-4.25%) of the Pt shell weaken the binding of oxygenated species, leading to enhanced ORR performance in fuel cells.
- Research Organization:
- Los Alamos National Lab. (LANL), Los Alamos, NM (United States); Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Sponsoring Organization:
- USDOE Office of Energy Efficiency and Renewable Energy (EERE), Sustainable Transportation Office. Hydrogen Fuel Cell Technologies Office; USDOE Office of Science (SC), Basic Energy Sciences (BES)
- Grant/Contract Number:
- AC52-06NA25396; AC02-06CH11357; 89233218CNA000001; AC05-00OR22725
- OSTI ID:
- 1591934
- Alternate ID(s):
- OSTI ID: 1494465; OSTI ID: 1531228
- Report Number(s):
- LA-UR-18-28949; S2542435118304549; PII: S2542435118304549
- Journal Information:
- Joule, Journal Name: Joule Vol. 3 Journal Issue: 1; ISSN 2542-4351
- Publisher:
- ElsevierCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Biaxial Strains Mediated Oxygen Reduction Electrocatalysis on Fenton Reaction Resistant L1 0 ‐PtZn Fuel Cell Cathode
Face-centered tetragonal (FCT) Fe and Co alloys of Pt as catalysts for the oxygen reduction reaction (ORR): A DFT study