Understanding Thermodynamic and Kinetic Contributions in Expanding the Stability Window of Aqueous Electrolytes
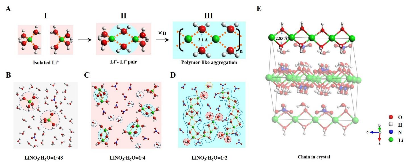
Aqueous electrolytes come with an intrinsic narrow electrochemical stability window (1.23 V). Expanding this window represents significant benefits in both fundamental science and practical battery applications. Recent break throughs made via super-concentration have resulted in >3.0 V windows, but fundamental understanding of the related mechanism is still absent. In the present work, we examined the widened window (2.55 V) of a super-concentrated (unsaturated) aqueous solution of LiNO3 through both theoretical and spectral analyses and discovered that a local structure of intimate Li+-water interaction arises at super-concentration, generating (Li+(H2O)2)n polymer-like chains to replace the ubiquitous hydrogen bonding between water molecules. Such structure is mainly responsible for the expanded electrochemical stability window. Further theoretical and experimental analyses quantitatively differentiate the contributions to this window, identifying the kinetic factor (desolvation) as the main contributor. Such molecular-level and quantitative understanding will further assist in tailor designing more effective approaches to stabilizing water electrochemically.
- Research Organization:
- Argonne National Lab. (ANL), Argonne, IL (United States)
- Sponsoring Organization:
- USDOE Office of Energy Efficiency and Renewable Energy (EERE)
- Grant/Contract Number:
- AC02-06CH11357
- OSTI ID:
- 1579296
- Alternate ID(s):
- OSTI ID: 1503575
- Journal Information:
- Chem, Journal Name: Chem Vol. 4 Journal Issue: 12; ISSN 2451-9294
- Publisher:
- ElsevierCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
“Water‐in‐Salt” Electrolyte Makes Aqueous Sodium‐Ion Battery Safe, Green, and Long‐Lasting
Electrodes with Electrodeposited Water-excluding Polymer Coating Enable High-Voltage Aqueous Supercapacitors