Polyelectrolyte Complexation of Oligonucleotides by Charged Hydrophobic—Neutral Hydrophilic Block Copolymers
- Univ. of Chicago, IL (United States)
- Univ. of Chicago, IL (United States); Argonne National Lab. (ANL), Lemont, IL (United States)
- Swarthmore College, PA (United States)
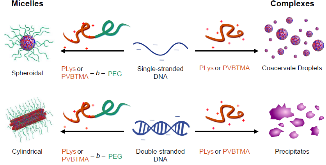
Polyelectrolyte complex micelles (PCMs, core-shell nanoparticles formed by complexation of a polyelectrolyte with a polyelectrolyte-hydrophilic neutral block copolymer) offer a solution to the critical problem of delivering therapeutic nucleic acids, Despite this, few systematic studies have been conducted on how parameters such as polycation charge density, hydrophobicity, and choice of charged group influence PCM properties, despite evidence that these strongly influence the complexation behavior of polyelectrolyte homopolymers. In this article, we report a comparison of oligonucleotide PCMs and polyelectrolyte complexes formed by poly(lysine) and poly((vinylbenzyl) trimethylammonium) (PVBTMA), a styrenic polycation with comparatively higher charge density, increased hydrophobicity, and a permanent positive charge. All of these differences have been individually suggested to provide increased complex stability, but we find that PVBTMA in fact complexes oligonucleotides more weakly than does poly(lysine), as measured by stability versus added salt. Using small angle X-ray scattering and electron microscopy, we find that PCMs formed from both cationic blocks exhibit very similar structure-property relationships, with PCM radius determined by the cationic block size and shape controlled by the hybridization state of the oligonucleotides. These observations narrow the design space for optimizing therapeutic PCMs and provide new insights into the rich polymer physics of polyelectrolyte self-assembly.
- Research Organization:
- Argonne National Laboratory (ANL), Argonne, IL (United States)
- Sponsoring Organization:
- National Institute of Standards and Technology (NIST) - Center for Hierarchical Materials Design (CHiMaD); USDOE Office of Science (SC), Basic Energy Sciences (BES) (SC-22). Materials Sciences & Engineering Division
- Grant/Contract Number:
- AC02-06CH11357
- OSTI ID:
- 1494914
- Journal Information:
- Polymers, Journal Name: Polymers Journal Issue: 1 Vol. 11; ISSN POLYCK; ISSN 2073-4360
- Publisher:
- MDPICopyright Statement
- Country of Publication:
- United States
- Language:
- English
Structural transitions and encapsulation selectivity of thermoresponsive polyelectrolyte complex micelles
|
journal | January 2019 |
Similar Records
Structure–Property Relationships of Oligonucleotide Polyelectrolyte Complex Micelles