Atomic-Scale Understanding of Catalyst Activation: Carboxylic Acid Solutions, but Not the Acid Itself, Increase the Reactivity of Anatase (001) Faceted Nanocatalysts
- Cornell Univ., Ithaca, NY (United States). Dept. of Chemistry and Chemical Biology
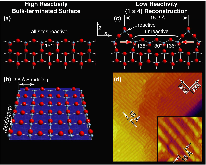
Our ability to predict nanocatalyst reactivity has been hindered by our lack of atomic-scale understanding of nanocatalyst surface structure. Do nanocatalyst surfaces adopt a bulk-terminated structure or do they reconstruct to minimize their free energy, thereby lowering their reactivity as often observed in vacuum? Similarly, do nanocatalysts processed at high temperatures maintain their low reactivity, reconstructed surfaces when used at low temperatures? Using a new technique for the preparation of anatase nanocatalysts suitable for atomic-scale imaging and surface spectroscopy, we show that solution-prepared anatase is terminated by a monolayer of fluorine, which acts as an atomic-scale oleophobic coating, preventing the accumulation of adventitious carbon. We further show that the most common TiO2 functionalization chemistry, a carboxylic acid solution, causes the spontaneous reorganization of a reconstructed anatase nanocatalyst, leading to a five-fold increase in reactive sites. This reorganization is not observed when carboxylic acids are deposited from the gas phase, suggesting that model experiments in vacuum environments can lead to a nonequilibrium, kinetically trapped state that may not be catalytically relevant. Aqueous carboxylic acid solutions produce densely packed carboxylate monolayers with richer adsorption geometries than previously predicted. Ab initio simulations show that although the carboxylate termination is somewhat less effective at removing surface stress than the reconstruction, it is more effective in lowering the surface energy. This observation suggests that bulk-terminated metal-oxide nanocrystals may be common in reactive environments, even if high temperatures are used to process the nanocatalyst or if the reactant is later rinsed off. As such, the assumption of a bulk-terminated surface may be a reasonable starting point for “materials-by-design” approaches to computationally engineered nanocatalysts.
- Research Organization:
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). National Energy Research Scientific Computing Center (NERSC)
- Sponsoring Organization:
- USDOE Office of Science (SC); National Science Foundation (NSF)
- Grant/Contract Number:
- AC02-05CH11231
- OSTI ID:
- 1483673
- Journal Information:
- Journal of Physical Chemistry. C, Vol. 122, Issue 8; ISSN 1932-7447
- Publisher:
- American Chemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Similar Records
Structure and Stability of Pristine and Carboxylate-Covered Anatase TiO2(001) in Aqueous Environment
Anatase and Rutile Surfaces with Adsorbates Representative of Acidic and Basic Conditions