Spontaneous Ionic Polarization in Ammonia-Based Ionic Liquid [Spontaneous Ionic Polarization in Ionic Liquid]
- Pohang Accelerator Lab., Pohang (South Korea); SLAC National Accelerator Lab., Menlo Park, CA (United States)
- SLAC National Accelerator Lab., Menlo Park, CA (United States)
- Stanford Univ., Stanford, CA (United States)
- SLAC National Accelerator Lab., Menlo Park, CA (United States); Stanford Univ., Stanford, CA (United States)
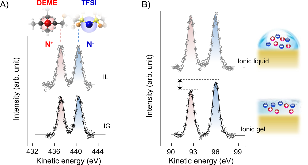
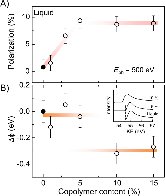
Ionic liquids and gels have attracted attention for a variety of energy storage applications, as well as for high performance electrolytes for batteries and super-capacitors. Although the electronic structure of ionic electrolytes in these applications is of practical importance for device design and improved performance, the understanding of the electronic structure of ionic liquids and gels is still at an early stage. Here we report soft x-ray spectroscopic measurements of the surface electronic structure of a representative ammonia-based ionic gel (DEME-TFSI with PSPMMA- PS copolymer). We observe that near the outermost surface, the area of the anion peak (1s N- core level in TFSI) is relatively larger than that of the cation peak (N+ in DEME). This spontaneous ionic polarization of the electrolyte surface, which is absent for the pure ionic liquid without copolymer, can be directly tuned by the copolymer content in the ionic gel, and further results in a modulation in work function. Finally, these results shed new light on the control of surface electronic properties of ionic electrolytes, as well as a difference between their implementation in ionic liquids and gels.
- Research Organization:
- SLAC National Accelerator Laboratory (SLAC), Menlo Park, CA (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES) (SC-22)
- Grant/Contract Number:
- AC02-76SF00515
- OSTI ID:
- 1452771
- Journal Information:
- ACS Applied Energy Materials, Journal Name: ACS Applied Energy Materials Journal Issue: 6 Vol. 1; ISSN 2574-0962
- Publisher:
- American Chemical Society (ACS)Copyright Statement
- Country of Publication:
- United States
- Language:
- English
Similar Records
Molecular-level insights into structure and dynamics in ionic liquids and polymer gel electrolytes
Phase Behavior and Ionic Conductivity of Concentrated Solutions of Polystyrene-Poly(ethylene oxide) Diblock Copolymers in an Ionic Liquid