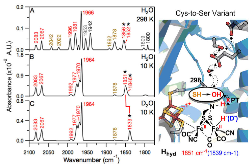
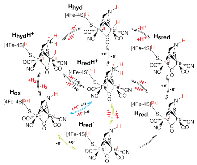
|
Identification and Characterization of the “Super-Reduced” State of the H-Cluster in [FeFe] Hydrogenase: A New Building Block for the Catalytic Cycle?
|
journal
|
October 2012 |
|
Artificial Maturation of the Highly Active Heterodimeric [FeFe] Hydrogenase from Desulfovibrio desulfuricans ATCC 7757
|
journal
|
August 2016 |
|
The active site of the [FeFe]-hydrogenase from Desulfovibrio desulfuricans. II. Redox properties, light sensitivity and CO-ligand exchange as observed by infrared spectroscopy
|
journal
|
December 2005 |
|
The structure and mechanism of iron-hydrogenases
|
journal
|
November 1990 |
|
A pulsed EPR study of redox-dependent hyperfine interactions for the nickel centre ofDesulfovibrio gigashydrogenase
|
journal
|
December 1988 |
|
Desulfovibrio desulfuricans iron hydrogenase: the structure shows unusual coordination to an active site Fe binuclear center
|
journal
|
January 1999 |
|
Molecular dynamics study of the proposed proton transport pathways in [FeFe]-hydrogenase
|
journal
|
January 2014 |
|
The effect of a C298D mutation in CaHydA [FeFe]-hydrogenase: Insights into the protein-metal cluster interaction by EPR and FTIR spectroscopic investigation
|
journal
|
January 2016 |
|
Roles of the F-domain in [FeFe] hydrogenase
|
journal
|
February 2018 |
|
[FeFe]- and [NiFe]-hydrogenase diversity, mechanism, and maturation
|
journal
|
June 2015 |
|
Diiron Azadithiolates as Models for the [FeFe]-Hydrogenase Active Site and Paradigm for the Role of the Second Coordination Sphere
|
journal
|
June 2015 |
|
Single-Amino Acid Modifications Reveal Additional Controls on the Proton Pathway of [FeFe]-Hydrogenase
|
journal
|
May 2016 |
|
Hydrogenase Enzymes and Their Synthetic Models: The Role of Metal Hydrides
|
journal
|
June 2016 |
|
Hybrid [FeFe]-Hydrogenases with Modified Active Sites Show Remarkable Residual Enzymatic Activity
|
journal
|
February 2015 |
|
Spectroelectrochemical Characterization of the Active Site of the [FeFe] Hydrogenase HydA1 from Chlamydomonas reinhardtii
|
journal
|
August 2009 |
|
Crystallographic and FTIR Spectroscopic Evidence of Changes in Fe Coordination Upon Reduction of the Active Site of the Fe-Only Hydrogenase from Desulfovibrio d esulfuricans
|
journal
|
February 2001 |
|
A Capable Bridging Ligand for Fe-Only Hydrogenase: Density Functional Calculations of a Low-Energy Route for Heterolytic Cleavage and Formation of Dihydrogen
|
journal
|
April 2001 |
|
Mechanistic and Physiological Implications of the Interplay among Iron–Sulfur Clusters in [FeFe]-Hydrogenases. A QM/MM Perspective
|
journal
|
November 2011 |
|
Catalytic Turnover of [FeFe]-Hydrogenase Based on Single-Molecule Imaging
|
journal
|
October 2011 |
|
EPR and FTIR Analysis of the Mechanism of H 2 Activation by [FeFe]-Hydrogenase HydA1 from Chlamydomonas reinhardtii
|
journal
|
April 2013 |
|
New Redox States Observed in [FeFe] Hydrogenases Reveal Redox Coupling Within the H-Cluster
|
journal
|
July 2014 |
|
Investigations on the Role of Proton-Coupled Electron Transfer in Hydrogen Activation by [FeFe]-Hydrogenase
|
journal
|
October 2014 |
|
Dithiomethylether as a Ligand in the Hydrogenase H-Cluster
|
journal
|
April 2008 |
|
Quantum Refinement of [FeFe] Hydrogenase Indicates a Dithiomethylamine Ligand
|
journal
|
April 2010 |
|
Identification of a Catalytic Iron-Hydride at the H-Cluster of [FeFe]-Hydrogenase
|
journal
|
December 2016 |
|
Proton Coupled Electronic Rearrangement within the H-Cluster as an Essential Step in the Catalytic Cycle of [FeFe] Hydrogenases
|
journal
|
January 2017 |
|
Direct Observation of an Iron-Bound Terminal Hydride in [FeFe]-Hydrogenase by Nuclear Resonance Vibrational Spectroscopy
|
journal
|
March 2017 |
|
Reduction Potentials of [FeFe]-Hydrogenase Accessory Iron–Sulfur Clusters Provide Insights into the Energetics of Proton Reduction Catalysis
|
journal
|
July 2017 |
|
Activation Thermodynamics and H/D Kinetic Isotope Effect of the H ox to H red H + Transition in [FeFe] Hydrogenase
|
journal
|
September 2017 |
|
Bridging Hydride at Reduced H-Cluster Species in [FeFe]-Hydrogenases Revealed by Infrared Spectroscopy, Isotope Editing, and Quantum Chemistry
|
journal
|
August 2017 |
|
Intercluster Redox Coupling Influences Protonation at the H-cluster in [FeFe] Hydrogenases
|
journal
|
October 2017 |
|
Reaction Coordinate Leading to H 2 Production in [FeFe]-Hydrogenase Identified by Nuclear Resonance Vibrational Spectroscopy and Density Functional Theory
|
journal
|
November 2017 |
|
Unique Spectroscopic Properties of the H-Cluster in a Putative Sensory [FeFe] Hydrogenase
|
journal
|
January 2018 |
|
Proton Transport in Clostridium pasteurianum [FeFe] Hydrogenase I: A Computational Study
|
journal
|
January 2014 |
|
Biomimetic assembly and activation of [FeFe]-hydrogenases
|
journal
|
June 2013 |
|
Spontaneous activation of [FeFe]-hydrogenases by an inorganic [2Fe] active site mimic
|
journal
|
August 2013 |
|
Accumulating the hydride state in the catalytic cycle of [FeFe]-hydrogenases
|
journal
|
July 2017 |
|
Vibrational spectroscopy reveals the initial steps of biological hydrogen evolution
|
journal
|
January 2016 |
|
Protonation/reduction dynamics at the [4Fe–4S] cluster of the hydrogen-forming cofactor in [FeFe]-hydrogenases
|
journal
|
January 2018 |
|
14N HYSCORE investigation of the H-cluster of [FeFe] hydrogenase: evidence for a nitrogen in the dithiol bridge
|
journal
|
January 2009 |
|
The organometallic active site of [Fe]hydrogenase: Models and entatic states
|
journal
|
March 2003 |
|
Importance of the Protein Framework for Catalytic Activity of [FeFe]-Hydrogenases
|
journal
|
November 2011 |
|
Utilization of Non-Innocent Redox Ligands in [FeFe] Hydrogenase Modeling for Hydrogen Production
|
journal
|
October 2015 |
|
Structure-function relationships among the nickel-containing hydrogenases
|
journal
|
February 1992 |
|
Merging [FeFe]-hydrogenases with materials and nanomaterials as biohybrid catalysts for solar H 2 production
|
conference
|
September 2007 |
|
X-ray Crystal Structure of the Fe-Only Hydrogenase (CpI) from Clostridium pasteurianum to 1.8 Angstrom Resolution
|
journal
|
December 1998 |
|
The Physiological Functions and Structural Determinants of Catalytic Bias in the [FeFe]-Hydrogenases CpI and CpII of Clostridium pasteurianum Strain W5
|
journal
|
July 2017 |