Evaluating Transport Properties and Ionic Dissociation of LiPF6 in Concentrated Electrolyte
- Argonne National Lab. (ANL), Argonne, IL (United States)
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). Environmental Technologies Area
- Pacific Northwest National Lab. (PNNL), Richland, WA (United States). Environmental Molecular Sciences Lab.
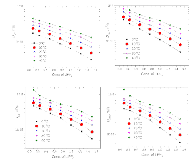
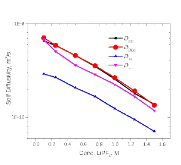
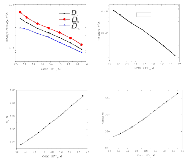
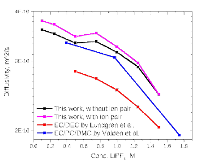
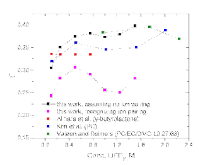
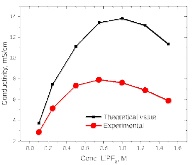
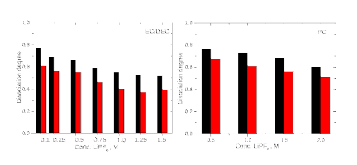
The presence of lithium hexafluorophosphate (LiPF6) ion pairs in carbonate-based electrolyte solutions is widely accepted in the field of battery electrolyte research and is expected to affect solution transport properties. No existing techniques are capable of directly quantifying salt dissociation in these solutions. Previous publications by others have provided estimates of dissociation degrees using dilute solution theory and pulsed field gradient nuclear magnetic resonance spectroscopy (PFG-NMR) measurements of self-diffusivity. However, the behavior of a concentrated electrolyte solution can deviate significantly from dilute solution theory predictions. This paper, for the first time, instead uses Onsager–Stefan–Maxwell concentrated solution theory and the generalized Darken relation with PFG-NMR measurements to quantify the degrees of dissociation in electrolyte solutions (LiPF6 in ethylene carbonate/diethyl carbonate, 1:1 by weight). At LiPF6 concentrations ranging from 0.1 M to 1.5 M, the salt dissociation degree is found to range from 61% to 37%. Finally, transport properties are then calculated through concentrated solution theory with corrections for these significant levels of ion pairing.
- Research Organization:
- Lawrence Berkeley National Laboratory (LBNL), Berkeley, CA (United States)
- Sponsoring Organization:
- USDOE Office of Energy Efficiency and Renewable Energy (EERE), Vehicle Technologies Office (EE-3V); USDOE Office of Science (SC), Biological and Environmental Research (BER) (SC-23)
- Grant/Contract Number:
- AC02-05CH11231; AC02-06CH11357
- OSTI ID:
- 1436335
- Journal Information:
- Journal of the Electrochemical Society, Journal Name: Journal of the Electrochemical Society Journal Issue: 12 Vol. 164; ISSN 0013-4651
- Publisher:
- The Electrochemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Time-dependent pair distribution functions based on Smoluchowski equation and application to an electrolyte solution
|
journal | March 2018 |
Fluorinated polysulfonamide based single ion conducting room temperature applicable gel-type polymer electrolytes for lithium ion batteries
|
journal | January 2019 |
Temperature and Concentration Dependence of the Ionic Transport Properties of Lithium-Ion Battery Electrolytes
|
journal | January 2019 |
Communication—Microscopic View of the Ethylene Carbonate Based Lithium-Ion Battery Electrolyte by X-ray Scattering
|
journal | January 2019 |
Similar Records
Anisotropic Ion Diffusion and Electrochemically Driven Transport in Nanostructured Block Copolymer Electrolytes