Hydride Conformers of the Nitrogenase FeMo-cofactor Two-Electron Reduced State E 2 (2H), Assigned Using Cryogenic Intra Electron Paramagnetic Resonance Cavity Photolysis
- Departments of Chemistry and Molecular Biosciences, Northwestern University, Evanston, Illinois 60208, United States
- Department of Chemistry and Biochemistry, Utah State University, Logan, Utah 84322, United States
- Department of Biochemistry, Virginia Polytechnic Institute and State University, Blacksburg, Virginia 24061, United States
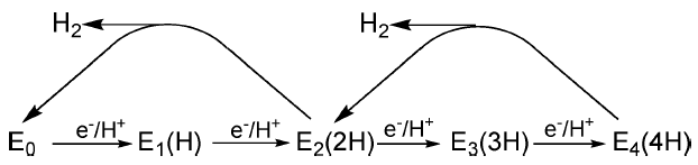
Early studies in which nitrogenase was freeze-trapped during enzymatic turnover revealed the presence of high-spin (S = 3/2) electron paramagnetic resonance (EPR) signals from the active-site FeMo-cofactor (FeMo-co) in electron-reduced intermediates of the MoFe protein. Historically denoted as 1b and 1c, each of the signals is describable as a fictitious spin system, S' = 1/2, with anisotropic g' tensor, 1b with g' = [4.21, 3.76, ?] and 1c with g' = [4.69, ~3.20, ?]. A clear discrepancy between the magnetic properties of 1b and 1c and the kinetic analysis of their appearance during pre-steady-state turnover left their identities in doubt, however. We subsequently associated 1b with the state having accumulated 2[e–/H+], denoted as E2(2H), and suggested that the reducing equivalents are stored on the catalytic FeMo-co cluster as an iron hydride, likely an [Fe–H–Fe] hydride bridge. Intra-EPR cavity photolysis (450 nm; temperature-independent from 4 to 12 K) of the E2(2H)/1b state now corroborates the identification of this state as storing two reducing equivalents as a hydride. Photolysis converts E2(2H)/1b to a state with the same EPR spectrum, and thus the same cofactor structure as pre-steady-state turnover 1c, but with a different active-site environment. Upon annealing of the photogenerated state at temperature T = 145 K, it relaxes back to E2(2H)/1b. This implies that the 1c signal comes from an E2(2H) hydride isomer of E2(2H)/1b that stores its two reducing equivalents either as a hydride bridge between a different pair of iron atoms or an Fe–H terminal hydride.
- Research Organization:
- Utah State Univ., Logan, UT (United States); Virginia Polytechnic Inst. and State Univ. (Virginia Tech), Blacksburg, VA (United States); Northwestern Univ., Evanston, IL (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); National Inst. of Health (NIH) (United States)
- Grant/Contract Number:
- SC0010687; SC0010834; GM111097
- OSTI ID:
- 1429515
- Alternate ID(s):
- OSTI ID: 1508781
- Journal Information:
- Inorganic Chemistry, Journal Name: Inorganic Chemistry Vol. 57 Journal Issue: 12; ISSN 0020-1669
- Publisher:
- American Chemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Web of Science
Carbon Dioxide Insertion into Bridging Iron Hydrides: Kinetic and Mechanistic Studies: Carbon Dioxide Insertion into Bridging Iron Hydrides: Kinetic and Mechanistic Studies
|
journal | February 2019 |
A model for dinitrogen binding in the E 4 state of nitrogenase
|
journal | January 2019 |
Critical computational analysis illuminates the reductive-elimination mechanism that activates nitrogenase for N 2 reduction
|
journal | October 2018 |
Similar Records
A conformational equilibrium in the nitrogenase MoFe protein with an α-V70I amino acid substitution illuminates the mechanism of H2 formation
Electron Redistribution within the Nitrogenase Active Site FeMo-cofactor During Reductive Elimination of H2 to Achieve N=N Triple-Bond Activation