Identification and Characterization of JAK2 Pseudokinase Domain Small Molecule Binders
Journal Article
·
· ACS Medicinal Chemistry Letters
- Yale Univ., New Haven, CT (United States)
- Tampere Univ. of Technology (Finland)
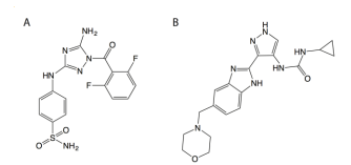
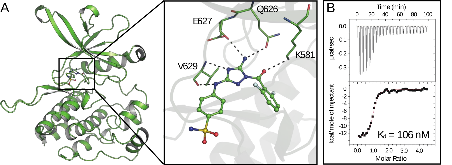
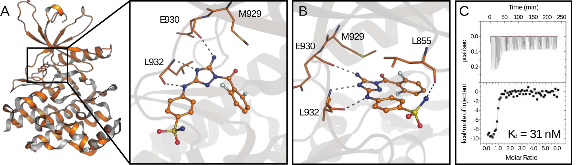
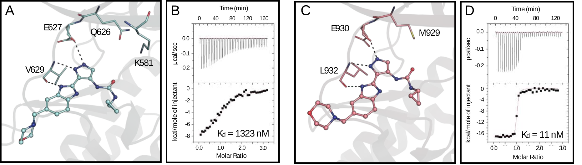
Janus kinases (JAKs) regulate hematopoiesis via the cytokine-mediated JAK-STAT signaling pathway. JAKs contain tandem C-terminal pseudokinase (JH2) and tyrosine kinase (JH1) domains. The JAK2 pseudokinase domain adopts a protein kinase fold and, despite its pseudokinase designation, binds ATP with micromolar affinity. Recent evidence shows that displacing ATP from the JAK2 JH2 domain alters the hyperactivation state of the oncogenic JAK2 V617F protein while sparing the wild type JAK2 protein. In this study, small molecule binders of JAK2 JH2 were identified via an in vitro screen. Top hits were characterized using biophysical and structural approaches. Development of pseudokinase-selective compounds may offer novel pharmacological opportunities for treating cancers driven by JAK2 V617F and other oncogenic JAK mutants.
- Research Organization:
- Argonne National Laboratory (ANL), Argonne, IL (US)
- Sponsoring Organization:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI ID:
- 1390880
- Journal Information:
- ACS Medicinal Chemistry Letters, Journal Name: ACS Medicinal Chemistry Letters Journal Issue: 6 Vol. 8; ISSN 1948-5875
- Publisher:
- American Chemical Society (ACS)Copyright Statement
- Country of Publication:
- United States
- Language:
- ENGLISH
Similar Records
Receptor-mediated dimerization of JAK2 FERM domains is required for JAK2 activation
Expression, purification, characterization and crystallization of non- and phosphorylated states of JAK2 and JAK3 kinase domain
Journal Article
·
Tue Jul 24 20:00:00 EDT 2018
· eLife
·
OSTI ID:1628888
Expression, purification, characterization and crystallization of non- and phosphorylated states of JAK2 and JAK3 kinase domain
Journal Article
·
Tue May 29 00:00:00 EDT 2012
· Protein Expres. Purif.
·
OSTI ID:1037899