|
A Biomimetic Model of the Electron Transfer between P680 and the TyrZ-His190 Pair of PSII
|
journal
|
February 2005 |
|
Concerted Proton–Electron Transfer in Pyridylphenols: The Importance of the Hydrogen Bond
|
journal
|
January 2008 |
|
Influence of the Protonic State of an Imidazole-Containing Ligand on the Electrochemical and Photophysical Properties of a Ruthenium(II)–Polypyridine-Type Complex
|
journal
|
October 2007 |
|
Photosystem II: The machinery of photosynthetic water splitting
|
journal
|
October 2008 |
|
Solvent relaxation dynamics and electron transfer
|
journal
|
October 1993 |
|
Electron transfers in chemistry and biology
|
journal
|
August 1985 |
|
Proton and hydrogen currents in photosynthetic water oxidation
|
journal
|
May 2000 |
|
Mechanism of photosynthetic water oxidation: combining biophysical studies of photosystem II with inorganic model chemistry
|
journal
|
January 2001 |
|
Electron paramagnetic resonance kinetic studies of the S states in spinach PSII membranes
|
journal
|
December 1997 |
|
Energy surfaces, reorganization energies, and coupling elements in electron transfer
|
journal
|
June 1999 |
|
pH dependence of charge transfer between tryptophan and tyrosine in dipeptides
|
journal
|
February 2005 |
|
Back-reactions, short-circuits, leaks and other energy wasteful reactions in biological electron transfer: Redox tuning to survive life in O 2
|
journal
|
January 2012 |
|
Janus-faced charge recombinations in photosystem II photoinhibition
|
journal
|
April 2009 |
|
Moving Protons and Electrons in Biomimetic Systems
|
journal
|
March 2015 |
|
Proton-Coupled Electron Transfer and a Tyrosine–Histidine Pair in a Photosystem II-Inspired β-Hairpin Maquette: Kinetics on the Picosecond Time Scale
|
journal
|
February 2016 |
|
Exploring Autoionization and Photoinduced Proton-Coupled Electron Transfer Pathways of Phenol in Aqueous Solution
|
journal
|
September 2015 |
|
Hydrogen-Bond Relays in Concerted Proton–Electron Transfers
|
journal
|
October 2011 |
|
Proton-Coupled Electron Transfer with Photoexcited Metal Complexes
|
journal
|
February 2013 |
|
Theory of Proton-Coupled Electron Transfer in Energy Conversion Processes
|
journal
|
December 2009 |
|
Mimicking Photosynthetic Solar Energy Transduction
|
journal
|
January 2001 |
|
Kinetics and Pathways of Charge Recombination in Photosystem II †
|
journal
|
July 2002 |
|
Use of 3-Aminotyrosine To Examine the Pathway Dependence of Radical Propagation in Escherichia coli Ribonucleotide Reductase
|
journal
|
December 2009 |
|
Proton/Hydrogen Transfer Affects the S-State-Dependent Microsecond Phases of P680 + Reduction during Water Splitting
|
journal
|
February 1998 |
|
Function of Tyrosine Z in Water Oxidation by Photosystem II: Electrostatical Promotor Instead of Hydrogen Abstractor †
|
journal
|
January 1998 |
|
The Role of Hydrogen Bonds for the Multiphasic P680 + • Reduction by Y Z in Photosystem II with Intact Oxyen Evolution Capacity. Analysis of Kinetic H/D Isotope Exchange Effects †
|
journal
|
February 1999 |
|
Role of D1-His190 in the Proton-Coupled Oxidation of Tyrosine Y Z in Manganese-Depleted Photosystem II †
|
journal
|
September 1999 |
|
Radical Initiation in the Class I Ribonucleotide Reductase: Long-Range Proton-Coupled Electron Transfer?
|
journal
|
June 2003 |
|
Update 1 of: Electrochemical Approach to the Mechanistic Study of Proton-Coupled Electron Transfer
|
journal
|
December 2010 |
|
Theory of Coupled Electron and Proton Transfer Reactions
|
journal
|
December 2010 |
|
Proton-Coupled Electron Transfer
|
journal
|
April 2012 |
|
Biochemistry and Theory of Proton-Coupled Electron Transfer
|
journal
|
January 2014 |
|
Chemical Redox Agents for Organometallic Chemistry
|
journal
|
January 1996 |
|
Manganese Cluster in Photosynthesis: Where Plants Oxidize Water to Dioxygen
|
journal
|
January 1996 |
|
Quantum Mechanical Continuum Solvation Models
|
journal
|
August 2005 |
|
Photochemistry of MLCT excited states. Effect of nonchromophoric ligand variations on photophysical properties in the series cis-Ru(bpy)2L22+
|
journal
|
August 1983 |
|
Chemical Approaches to Artificial Photosynthesis. 2
|
journal
|
October 2005 |
|
Synthesis of Phosphonic Acid Derivatized Bipyridine Ligands and Their Ruthenium Complexes
|
journal
|
October 2013 |
|
Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields
|
journal
|
November 1994 |
|
Measurement of the extinction coefficient of the methyl viologen cation radical and the efficiency of its formation by semiconductor photocatalysis
|
journal
|
July 1982 |
|
Comparison of substituent effects on dissociation and conjugation of phenols with those of carboxylic acids in acetonitrile, N,N-dimethylformamide, and dimethyl sulfoxide
|
journal
|
June 1976 |
|
Electron ejection and electron capture by phenolic compounds
|
journal
|
January 1973 |
|
Substituent effects on the stabilities of phenoxyl radicals and the acidities of phenoxyl radical cations
|
journal
|
February 1991 |
|
Electron-Transfer Reactions of Ruthenium Trisbipyridyl-Viologen Donor-Acceptor Molecules: Comparison of the Distance Dependence of Electron Transfer-Rates in the Normal and Marcus Inverted Regions
|
journal
|
June 1994 |
|
Construction of Persistent Phenoxyl Radical with Intramolecular Hydrogen Bonding
|
journal
|
March 2001 |
|
Kinetics of reduction of eight viologens by dithionite ion
|
journal
|
May 1985 |
|
Laser flash spectroscopy of tris(2,2'-bipyridine)ruthenium(II) in solution
|
journal
|
June 1976 |
|
Measurement of rates of electron transfer between tris(2,2'-bipyridine)ruthenium(3+) and tris(1,10-phenanthroline)iron(2+) ions and between tris(1,10-phenanthroline)ruthenium(3+) and tris(2,2'-bipyridine)ruthenium(2+) ions by differential excitation flash photolysis
|
journal
|
April 1977 |
|
Normal and Inverse Primary Kinetic Deuterium Isotope Effects for C−H Bond Reductive Elimination and Oxidative Addition Reactions of Molybdenocene and Tungstenocene Complexes: Evidence for Benzene σ-Complex Intermediates
|
journal
|
February 2003 |
|
Concerted Proton−Electron Transfer in the Oxidation of Hydrogen-Bonded Phenols
|
journal
|
April 2006 |
|
Electron Transfer Reactions of Fluorotyrosyl Radicals
|
journal
|
October 2006 |
|
Concerted Proton−Electron Transfer Reactions in Water. Are the Driving Force and Rate Constant Depending on pH When Water Acts as Proton Donor or Acceptor?
|
journal
|
May 2007 |
|
Excited-State Quenching by Proton-Coupled Electron Transfer
|
journal
|
June 2007 |
|
Adiabatic and Non-adiabatic Concerted Proton−Electron Transfers. Temperature Effects in the Oxidation of Intramolecularly Hydrogen-Bonded Phenols
|
journal
|
August 2007 |
|
Buffer-Assisted Proton-Coupled Electron Transfer in a Model Rhenium−Tyrosine Complex
|
journal
|
September 2007 |
|
Proton-Coupled Electron Transfer of Tyrosine Oxidation: Buffer Dependence and Parallel Mechanisms
|
journal
|
December 2007 |
|
PELDOR Spectroscopy with DOPA-β2 and NH 2 Y-α2s: Distance Measurements between Residues Involved in the Radical Propagation Pathway of E. coli Ribonucleotide Reductase
|
journal
|
December 2007 |
|
A Hot Oxidant, 3-NO 2 Y 122 Radical, Unmasks Conformational Gating in Ribonucleotide Reductase
|
journal
|
November 2010 |
|
Proton-Coupled Electron Transfer from Tyrosine: A Strong Rate Dependence on Intramolecular Proton Transfer Distance
|
journal
|
August 2011 |
|
Proton Coupled Electron Transfer and Redox-Active Tyrosine Z in the Photosynthetic Oxygen-Evolving Complex
|
journal
|
July 2011 |
|
Kinetic Effects of Increased Proton Transfer Distance on Proton-Coupled Oxidations of Phenol-Amines
|
journal
|
November 2011 |
|
Influence of Donor–Acceptor Distance Variation on Photoinduced Electron and Proton Transfer in Rhenium(I)–Phenol Dyads
|
journal
|
July 2012 |
|
Spanning Four Mechanistic Regions of Intramolecular Proton-Coupled Electron Transfer in a Ru(bpy) 3 2+ –Tyrosine Complex
|
journal
|
September 2012 |
|
Multiple-Site Concerted Proton–Electron Transfer Reactions of Hydrogen-Bonded Phenols Are Nonadiabatic and Well Described by Semiclassical Marcus Theory
|
journal
|
September 2012 |
|
Proton-Coupled Electron Transfers: pH-Dependent Driving Forces? Fundamentals and Artifacts
|
journal
|
September 2013 |
|
The Rate Ladder of Proton-Coupled Tyrosine Oxidation in Water: A Systematic Dependence on Hydrogen Bonds and Protonation State
|
journal
|
June 2008 |
|
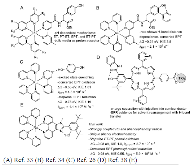
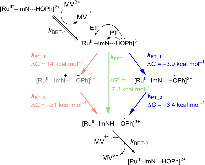
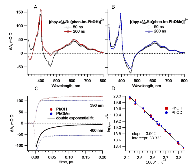
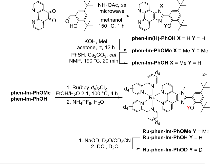
A Bioinspired Construct That Mimics the Proton Coupled Electron Transfer between P680 •+ and the Tyr Z -His190 Pair of Photosystem II
|
journal
|
August 2008 |
|
Proton-Coupled Electron Transfer in Photosystem II: Proton Inventory of a Redox Active Tyrosine
|
journal
|
August 2009 |
|
Isotope Effects on Hydride Transfer Reactions from Transition Metal Hydrides to Trityl Cation. An Inverse Isotope Effect for a Hydride Transfer
|
journal
|
April 1999 |
|
EPR Detection of the Transient Tyrosyl Radical in DNA Photolyase from Anacystis nidulans
|
journal
|
September 1999 |
|
Demand-Based Thiolate Anion Generation under Virtually Neutral Conditions: Influence of Steric and Electronic Factors on Chemo- and Regioselective Cleavage of Aryl Alkyl Ethers †
|
journal
|
September 2002 |
|
Proton-Coupled Electron Transfer Involving Tyrosine Z in Photosystem II †
|
journal
|
August 2002 |
|
Electron Transfer Driven by Proton Fluctuations in a Hydrogen-Bonded Donor−Acceptor Assembly
|
journal
|
June 2006 |
|
Proton-Coupled Electron Transfer and Tyrosine D of Photosystem II
|
journal
|
October 2007 |
|
Effects of Protonation State on a Tyrosine−Histidine Bioinspired Redox Mediator †
|
journal
|
November 2010 |
|
Broadband Spectral Probing Revealing Ultrafast Photochemical Branching after Ultraviolet Excitation of the Aqueous Phenolate Anion
|
journal
|
April 2011 |
|
Probing Quantum and Dynamic Effects in Concerted Proton–Electron Transfer Reactions of Phenol–Base Compounds
|
journal
|
December 2011 |
|
Proton-Coupled Electron Transfer in Tyrosine and a β-Hairpin Maquette: Reaction Dynamics on the Picosecond Time Scale
|
journal
|
August 2014 |
|
Analysis of Kinetic Isotope Effects for Proton-Coupled Electron Transfer Reactions †
|
journal
|
March 2009 |
|
The Kinetic Effect of Internal Hydrogen Bonds on Proton-Coupled Electron Transfer from Phenols: A Theoretical Analysis with Modeling of Experimental Data
|
journal
|
December 2009 |
|
Contemporary Issues in Electron Transfer Research
|
journal
|
January 1996 |
|
Proton-Coupled Electron Transfer Reactions: Evaluation of Rate Constants
|
journal
|
January 1996 |
|
Evaluation of Solvent Effects in Isotropic and Anisotropic Dielectrics and in Ionic Solutions with a Unified Integral Equation Method: Theoretical Bases, Computational Implementation, and Numerical Applications
|
journal
|
December 1997 |
|
New applications of integral equations methods for solvation continuum models: ionic solutions and liquid crystals
|
journal
|
January 1998 |
|
Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å
|
journal
|
April 2011 |
|
A bioinspired redox relay that mimics radical interactions of the Tyr–His pairs of photosystem II
|
journal
|
February 2014 |
|
A phenol–imidazole pro-ligand that can exist as a phenoxyl radical, alone and when complexed to copper( ii ) and zinc( ii )
|
journal
|
January 2003 |
|
Steric effect for proton, hydrogen-atom, and hydride transfer reactions with geometric isomers of NADH–model ruthenium complexes
|
journal
|
January 2012 |
|
Proton-coupled electron transfer: classification scheme and guide to theoretical methods
|
journal
|
January 2012 |
|
Photochemical tyrosine oxidation with a hydrogen-bonded proton acceptor by bidirectional proton-coupled electron transfer
|
journal
|
January 2012 |
|
Controlling electron transfer in strong time-dependent fields: Theory beyond the Golden Rule approximation
|
journal
|
December 2000 |
|
Dielectric Relaxation in Alkylcyanides
|
journal
|
August 1964 |
|
Multidimensional treatment of stochastic solvent dynamics in photoinduced proton-coupled electron transfer processes: Sequential, concerted, and complex branching mechanisms
|
journal
|
October 2011 |
|
Ab initio effective core potentials for molecular calculations. Potentials for K to Au including the outermost core orbitals
|
journal
|
January 1985 |
|
Dynamical effects in electron transfer reactions
|
journal
|
May 1986 |
|
Dynamic solvent effects on outer‐sphere electron transfer
|
journal
|
August 1987 |
|
Outer sphere electron transfer in polar solvents. Activationless and inverted regimes
|
journal
|
December 1987 |
|
A new mixing of Hartree–Fock and local density‐functional theories
|
journal
|
January 1993 |
|
Density‐functional thermochemistry. III. The role of exact exchange
|
journal
|
April 1993 |
|
A new integral equation formalism for the polarizable continuum model: Theoretical background and applications to isotropic and anisotropic dielectrics
|
journal
|
August 1997 |
|
Kinetics and mechanism of electron transfer in intact photosystem II and in the isolated reaction center: Pheophytin is the primary electron acceptor
|
journal
|
April 2006 |
|
Probing concerted proton-electron transfer in phenol-imidazoles
|
journal
|
January 2008 |
|
The electrochemical approach to concerted proton--electron transfers in the oxidation of phenols in water
|
journal
|
October 2009 |
|
Improving the efficiency of water splitting in dye-sensitized solar cells by using a biomimetic electron transfer mediator
|
journal
|
April 2012 |
|
Mimicking the electron transfer chain in photosystem II with a molecular triad thermodynamically capable of water oxidation
|
journal
|
May 2012 |
|
Picosecond kinetics of fluorescence and absorbance changes in photosystem II particles excited at low photon density
|
journal
|
December 1987 |
|
Chemical complementation identifies a proton acceptor for redox-active tyrosine D in photosystem II
|
journal
|
December 1997 |
|
Intraprotein electron transfer between tyrosine and tryptophan in DNA photolyase from Anacystis nidulans
|
journal
|
May 1999 |
|
Density-functional exchange-energy approximation with correct asymptotic behavior
|
journal
|
September 1988 |
|
Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density
|
journal
|
January 1988 |
|
COOPERATION OF CHARGES IN PHOTOSYNTHETIC O 2 EVOLUTION–I. A LINEAR FOUR STEP MECHANISM
|
journal
|
June 1970 |
|
Photosynthetic O2 Formation Tracked by Time-Resolved X-ray Experiments
|
journal
|
November 2005 |
|
Polar Solvent Dynamics and Electron-Transfer Reactions
|
journal
|
March 1989 |
|
Photosystem II: The Reaction Center of Oxygenic Photosynthesis
|
journal
|
June 2013 |
|
Electron Transfer Reactions in Condensed Phases
|
journal
|
October 1984 |