Identification of Surface-Exposed Protein Radicals and A Substrate Oxidation Site in A-Class Dye-Decolorizing Peroxidase from Thermomonospora curvata
- Kansas State Univ., Manhattan, KS (United States). Dept. of Chemistry
- Kansas State Univ., Manhattan, KS (United States). Dept. of Biochemistry and Molecular Biophysics
- Florida State Univ., Tallahassee, FL (United States). National High Magnetic Field Lab. (MagLab)
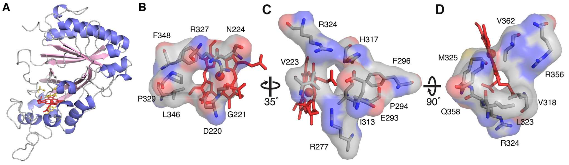
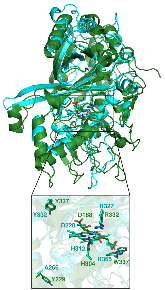
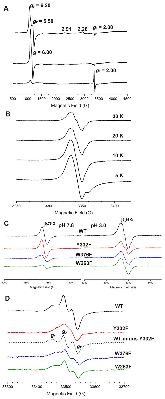
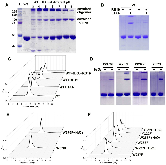
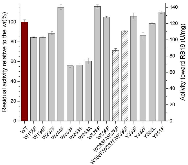
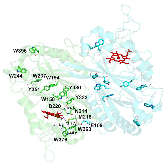
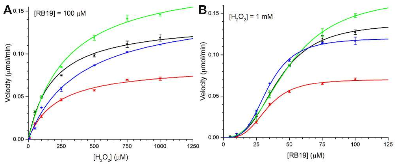
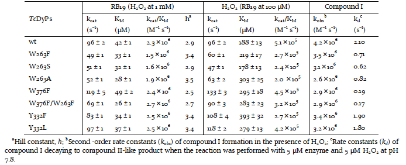
Dye-decolorizing peroxidases (DyPs) are a family of heme peroxidases in which a catalytic distal aspartate is involved in H2O2 activation to catalyze oxidations under acidic conditions. They have received much attention due to their potential applications in lignin compound degradation and biofuel production from biomass. However, the mode of oxidation in bacterial DyPs remains unknown. We have recently reported that the bacterial TcDyP from Thermomonospora curvata is among the most active DyPs and shows activity toward phenolic lignin model compounds. On the basis of the X-ray crystal structure solved at 1.75 Å, sigmoidal steady-state kinetics with Reactive Blue 19 (RB19), and formation of compound II like product in the absence of reducing substrates observed with stopped-flow spectroscopy and electron paramagnetic resonance (EPR), we hypothesized that the TcDyP catalyzes oxidation of large-size substrates via multiple surface-exposed protein radicals. Among 7 tryptophans and 3 tyrosines in TcDyP consisting of 376 residues for the matured protein, W263, W376, and Y332 were identified as surface-exposed protein radicals. Only the W263 was also characterized as one of the surface-exposed oxidation sites. SDS-PAGE and size-exclusion chromatography demonstrated that W376 represents an off-pathway destination for electron transfer, resulting in the cross-linking of proteins in the absence of substrates. Mutation of W376 improved compound I stability and overall catalytic efficiency toward RB19. While Y332 is highly conserved across all four classes of DyPs, its catalytic function in A-class TcDyP is minimal, possibly due to its extremely small solvent-accessible areas. Identification of surface-exposed protein radicals and substrate oxidation sites is important for understanding the DyP mechanism and modulating its catalytic functions for improved activity on phenolic lignin.
- Research Organization:
- Advanced Photon Source (APS), Argonne National Laboratory (ANL), Argonne, IL (US)
- Sponsoring Organization:
- USDOE
- OSTI ID:
- 1335976
- Journal Information:
- ACS Catalysis, Journal Name: ACS Catalysis Journal Issue: 12 Vol. 6; ISSN 2155-5435
- Publisher:
- American Chemical Society (ACS)Copyright Statement
- Country of Publication:
- United States
- Language:
- ENGLISH
Similar Records
Mechanistic Insights into Dye-Decolorizing Peroxidase Revealed by Solvent Isotope and Viscosity Effects
Complete genome sequence of Thermomonospora curvata type strain (B9)