Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte
Abstract
Fuel cells are attractive devices that convert chemical energy into electricity through the direct electrochemical reaction of hydrogen and oxygen. Intermediate temperature fuel cells operated at 200–300°C can simplify water and thermal managements, enable the use of non-precious or low-loading precious metal catalysts and provide insensitivity toward fuel and air impurities such as carbon monoxide. However, the performance of current intermediate temperature fuel cells is poor due to a lack of highly-conductive membrane electrolytes and optimal electrodes designed for these fuel cells. We demonstrate high-performing intermediate temperature fuel cells that use SnP2O7–polymer composite membranes and a quaternary ammonium-biphosphate ion-pair coordinated polymer electrolyte in the electrodes. The peak power density of the fuel cell under H2 and O2 reached 870 mW cm-2 at 240°C with minimal performance loss under exposure to 25% carbon monoxide.
- Authors:
-
- Los Alamos National Lab. (LANL), Los Alamos, NM (United States)
- Ceramatec, Inc., Salt Lake City, UT (United States)
- Publication Date:
- Research Org.:
- Los Alamos National Laboratory (LANL), Los Alamos, NM (United States)
- Sponsoring Org.:
- USDOE Advanced Research Projects Agency - Energy (ARPA-E); USDOE National Nuclear Security Administration (NNSA)
- Contributing Org.:
- Univ. of Tennessee, Knoxville, TN (United States)
- OSTI Identifier:
- 1473810
- Alternate Identifier(s):
- OSTI ID: 1434125
- Report Number(s):
- LA-UR-17-31199
Journal ID: ISSN 1754-5692; EESNBY
- Grant/Contract Number:
- AC52-06NA25396; AR0000314
- Resource Type:
- Journal Article: Accepted Manuscript
- Journal Name:
- Energy & Environmental Science
- Additional Journal Information:
- Journal Volume: 11; Journal Issue: 4; Journal ID: ISSN 1754-5692
- Publisher:
- Royal Society of Chemistry
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 30 DIRECT ENERGY CONVERSION; 36 MATERIALS SCIENCE
Citation Formats
Lee, Kwan-Soo, Maurya, Sandip, Kim, Yu Seung, Kreller, Cortney R., Wilson, Mahlon S., Larsen, Dennis, Elangovan, S. Elango, and Mukundan, Rangachary. Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte. United States: N. p., 2018.
Web. doi:10.1039/C7EE03595K.
Lee, Kwan-Soo, Maurya, Sandip, Kim, Yu Seung, Kreller, Cortney R., Wilson, Mahlon S., Larsen, Dennis, Elangovan, S. Elango, & Mukundan, Rangachary. Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte. United States. https://doi.org/10.1039/C7EE03595K
Lee, Kwan-Soo, Maurya, Sandip, Kim, Yu Seung, Kreller, Cortney R., Wilson, Mahlon S., Larsen, Dennis, Elangovan, S. Elango, and Mukundan, Rangachary. 2018.
"Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte". United States. https://doi.org/10.1039/C7EE03595K. https://www.osti.gov/servlets/purl/1473810.
@article{osti_1473810,
title = {Intermediate temperature fuel cells via an ion-pair coordinated polymer electrolyte},
author = {Lee, Kwan-Soo and Maurya, Sandip and Kim, Yu Seung and Kreller, Cortney R. and Wilson, Mahlon S. and Larsen, Dennis and Elangovan, S. Elango and Mukundan, Rangachary},
abstractNote = {Fuel cells are attractive devices that convert chemical energy into electricity through the direct electrochemical reaction of hydrogen and oxygen. Intermediate temperature fuel cells operated at 200–300°C can simplify water and thermal managements, enable the use of non-precious or low-loading precious metal catalysts and provide insensitivity toward fuel and air impurities such as carbon monoxide. However, the performance of current intermediate temperature fuel cells is poor due to a lack of highly-conductive membrane electrolytes and optimal electrodes designed for these fuel cells. We demonstrate high-performing intermediate temperature fuel cells that use SnP2O7–polymer composite membranes and a quaternary ammonium-biphosphate ion-pair coordinated polymer electrolyte in the electrodes. The peak power density of the fuel cell under H2 and O2 reached 870 mW cm-2 at 240°C with minimal performance loss under exposure to 25% carbon monoxide.},
doi = {10.1039/C7EE03595K},
url = {https://www.osti.gov/biblio/1473810},
journal = {Energy & Environmental Science},
issn = {1754-5692},
number = 4,
volume = 11,
place = {United States},
year = {Thu Mar 01 00:00:00 EST 2018},
month = {Thu Mar 01 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
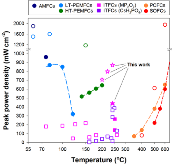 Fig. 1: Comparison of different type of fuel cells; filled symbol: H2/air; unfilled: H2/O2. Fuel cell data are taken from references: AMFCs5, 31; LT-PEMFCs4, 32; HT-PEMFCs33; SAFCs12; ITFCs16, 34; PCFCs and SOFCs6, 34.
Fig. 1: Comparison of different type of fuel cells; filled symbol: H2/air; unfilled: H2/O2. Fuel cell data are taken from references: AMFCs5, 31; LT-PEMFCs4, 32; HT-PEMFCs33; SAFCs12; ITFCs16, 34; PCFCs and SOFCs6, 34.
Works referenced in this record:
High-Temperature Proton-Exchange-Membrane Fuel Cells Using an Ether-Containing Polybenzimidazole Membrane as Electrolyte
journal, April 2012
- Li, Jin; Li, Xiaojin; Zhao, Yun
- ChemSusChem, Vol. 5, Issue 5
Ion Conducting Membranes for Fuel Cells and other Electrochemical Devices
journal, September 2013
- Kreuer, Klaus-Dieter
- Chemistry of Materials, Vol. 26, Issue 1
Ammonium Polyphosphate Composite Based Electrolytes for Intermediate Temperature Fuel Cells
journal, March 2013
- Kluy, N.; Reeb, B. B. L.; Paschos, O.
- ECS Transactions, Vol. 50, Issue 2
Proton conduction in acceptor doped SnP2O7
journal, February 2011
- Phadke, Satyajit R.; Bowers, Clifford R.; Wachsman, Eric D.
- Solid State Ionics, Vol. 183, Issue 1
Thin-Membrane Solid-Acid Fuel Cell
journal, May 2005
- Uda, T.; Haile, S. M.
- Electrochemical and Solid-State Letters, Vol. 8, Issue 5
Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries
journal, May 2011
- Jayaprakash, N.; Shen, J.; Moganty, Surya S.
- Angewandte Chemie, Vol. 123, Issue 26, p. 6026-6030
Lowering the Temperature of Solid Oxide Fuel Cells
journal, November 2011
- Wachsman, E. D.; Lee, K. T.
- Science, Vol. 334, Issue 6058
Origin of Toughness in Dispersion-Cast Nafion Membranes
journal, March 2015
- Kim, Yu Seung; Welch, Cynthia F.; Hjelm, Rex P.
- Macromolecules, Vol. 48, Issue 7
High Temperature, Low Humidity Proton Exchange Membrane Based on an Inorganic–Organic Hybrid Structure
journal, January 2010
- Jin, Yongcheng; Fujiwara, Keisuke; Hibino, Takashi
- Electrochemical and Solid-State Letters, Vol. 13, Issue 2
Structural investigation of ternary La/alkaline earth phosphate (La(1−x)MxP3Oy) (M=Ba, Ca, Sr) glasses
journal, June 2009
- Harley, Gabriel; Kreuer, Klaus-Dieter; Maier, Joachim
- Journal of Non-Crystalline Solids, Vol. 355, Issue 16-17
High-Power SOFC Using La[sub 0.9]Sr[sub 0.1]Ga[sub 0.8]Mg[sub 0.2]O[sub 3−δ]∕Ce[sub 0.8]Sm[sub 0.2]O[sub 2−δ] Composite Film
journal, January 2005
- Yan, Jingwang; Matsumoto, Hiroshige; Enoki, Makiko
- Electrochemical and Solid-State Letters, Vol. 8, Issue 8
Benzene Adsorption: A Significant Inhibitor for the Hydrogen Oxidation Reaction in Alkaline Conditions
journal, September 2017
- Matanovic, Ivana; Chung, Hoon Taek; Kim, Yu Seung
- The Journal of Physical Chemistry Letters, Vol. 8, Issue 19
Approaches and Recent Development of Polymer Electrolyte Membranes for Fuel Cells Operating above 100 °C
journal, December 2003
- Li, Qingfeng; He, Ronghuan; Jensen, Jens Oluf
- Chemistry of Materials, Vol. 15, Issue 26
Intermediate temperature stable proton conductors based upon SnP2O7, including additional H3PO4
journal, January 2010
- Xu, Xiaoxiang; Tao, Shanwen; Wormald, Philip
- Journal of Materials Chemistry, Vol. 20, Issue 36
Transformation of Rusty Stainless-Steel Meshes into Stable, Low-Cost, and Binder-Free Cathodes for High-Performance Potassium-Ion Batteries
journal, June 2017
- Zhu, Yun-hai; Yin, Yan-bin; Yang, Xu
- Angewandte Chemie International Edition, Vol. 56, Issue 27
Readily processed protonic ceramic fuel cells with high performance at low temperatures
journal, July 2015
- Duan, C.; Tong, J.; Shang, M.
- Science, Vol. 349, Issue 6254
Mesoporous lanthanum phosphate nanostructures containing H3PO4 as superior electrolyte for PEM fuel cells
journal, January 2013
- Chai, Zhanli; Suo, Quanyu; Wang, Hui
- RSC Advances, Vol. 3, Issue 44
An operationally flexible fuel cell based on quaternary ammonium-biphosphate ion pairs
journal, August 2016
- Lee, Kwan-Soo; Spendelow, Jacob S.; Choe, Yoong-Kee
- Nature Energy, Vol. 1, Issue 9, Article No. 16120
A Low-Operating-Temperature Solid Oxide Fuel Cell in Hydrocarbon-Air Mixtures
journal, June 2000
- Hibino, T.
- Science, Vol. 288, Issue 5473
CsH 2 PO 4 /Polyvinylidene Fluoride Composite Electrolytes for Intermediate Temperature Fuel Cells
journal, January 2014
- Qing, Geletu; Kikuchi, Ryuji; Takagaki, Atsushi
- Journal of The Electrochemical Society, Vol. 161, Issue 4
High-Temperature Polybenzimidazole Fuel Cell Membranes via a Sol−Gel Process
journal, September 2005
- Xiao, Lixiang; Zhang, Haifeng; Scanlon, Eugene
- Chemistry of Materials, Vol. 17, Issue 21
High-Performance Solid Acid Fuel Cells Through Humidity Stabilization
journal, January 2004
- Boysen, D. A.
- Science, Vol. 303, Issue 5654
Intermediate-Temperature Fuel Cell Employing CsH[sub 2]PO[sub 4]∕SiP[sub 2]O[sub 7]-Based Composite Electrolytes
journal, January 2006
- Matsui, Toshiaki; Kukino, Tomokazu; Kikuchi, Ryuji
- Journal of The Electrochemical Society, Vol. 153, Issue 2
Nanoporous alumina membranes filled with solid acid for thin film fuel cells at intermediate temperatures
journal, September 2004
- Bocchetta, P.; Chiavarotti, G.; Masi, R.
- Electrochemistry Communications, Vol. 6, Issue 9
Proton Conduction in In[sup 3+]-Doped SnP[sub 2]O[sub 7] at Intermediate Temperatures
journal, January 2006
- Nagao, Masahiro; Kamiya, Toshio; Heo, Pilwon
- Journal of The Electrochemical Society, Vol. 153, Issue 8
Proton conduction in metal pyrophosphates (MP2O7) at intermediate temperatures
journal, January 2010
- Jin, Yongcheng; Shen, Yanbai; Hibino, Takashi
- Journal of Materials Chemistry, Vol. 20, Issue 30
Stability and Conductivity of In3+-Doped SnP2O7 with Varying Phosphorous to Metal Ratios
journal, January 2013
- Kreller, C. R.; Wilson, M. S.; Mukundan, R.
- ECS Electrochemistry Letters, Vol. 2, Issue 9
Beyond catalysis and membranes: visualizing and solving the challenge of electrode water accumulation and flooding in AEMFCs
journal, January 2018
- Omasta, Travis J.; Park, Andrew M.; LaManna, Jacob M.
- Energy & Environmental Science, Vol. 11, Issue 3
A Flexible and Wearable Lithium-Oxygen Battery with Record Energy Density achieved by the Interlaced Architecture inspired by Bamboo Slips
journal, August 2016
- Liu, Qing-Chao; Liu, Tong; Liu, Da-Peng
- Advanced Materials, Vol. 28, Issue 38
Cation–Hydroxide–Water Coadsorption Inhibits the Alkaline Hydrogen Oxidation Reaction
journal, October 2016
- Chung, Hoon Taek; Martinez, Ulises; Matanovic, Ivana
- The Journal of Physical Chemistry Letters, Vol. 7, Issue 22
Conductivity of SnP2O7 and In-doped SnP2O7 prepared by an aqueous solution method
journal, March 2009
- Tao, Shanwen
- Solid State Ionics, Vol. 180, Issue 2-3
Advanced Electrodes for Solid Acid Fuel Cells by Platinum Deposition on CsH 2 PO 4
journal, April 2011
- Papandrew, Alexander B.; Chisholm, Calum R. I.; Elgammal, Ramez A.
- Chemistry of Materials, Vol. 23, Issue 7
Effect of Organic Cations on Hydrogen Oxidation Reaction of Carbon Supported Platinum
journal, January 2016
- Chung, Hoon Taek; Choe, Yoong-Kee; Martinez, Ulises
- Journal of The Electrochemical Society, Vol. 163, Issue 14
An Ultrathin Self-Humidifying Membrane for PEM Fuel Cell Application: Fabrication, Characterization, and Experimental Analysis
journal, July 2006
- Zhu, Xiaobing; Zhang, Huamin; Zhang, Yu
- The Journal of Physical Chemistry B, Vol. 110, Issue 29
CO tolerance and CO oxidation at Pt and Pt–Ru anode catalysts in fuel cell with polybenzimidazole–H3PO4 membrane
journal, August 2010
- Modestov, A. D.; Tarasevich, M. R.; Filimonov, V. Ya.
- Electrochimica Acta, Vol. 55, Issue 20
Proton conduction in non-doped and acceptor-doped metal pyrophosphate (MP2O7) composite ceramics at intermediate temperatures
journal, January 2012
- Sato, Yousuke; Shen, Yanbai; Nishida, Masakazu
- Journal of Materials Chemistry, Vol. 22, Issue 9
The effects of battlefield contaminants on PEMFC performance
journal, February 2000
- Moore, Jon M.; Adcock, Paul L.; Lakeman, J. Barry
- Journal of Power Sources, Vol. 85, Issue 2
The Membrane–Electrode Interface in PEFCs
journal, January 2007
- Pivovar, B. S.; Kim, Y. S.
- Journal of The Electrochemical Society, Vol. 154, Issue 8
Intragranular Phase Proton Conduction in Crystalline Sn 1– x In x P 2 O 7 ( x = 0 and 0.1)
journal, October 2017
- Kreller, Cortney R.; Pham, Hieu H.; Wilson, Mahlon S.
- The Journal of Physical Chemistry C, Vol. 121, Issue 43
Nanocrack-regulated self-humidifying membranes
journal, April 2016
- Park, Chi Hoon; Lee, So Young; Hwang, Doo Sung
- Nature, Vol. 532, Issue 7600
Anion exchange membrane fuel cells: Current status and remaining challenges
journal, January 2018
- Gottesfeld, Shimshon; Dekel, Dario R.; Page, Miles
- Journal of Power Sources, Vol. 375
Temperature and humidity dependence of the electrode polarization in intermediate-temperature fuel cells employing CsH2PO4/SiP2O7-based composite electrolytes
journal, May 2008
- Yoshimi, Shuichi; Matsui, Toshiaki; Kikuchi, Ryuji
- Journal of Power Sources, Vol. 179, Issue 2
Proton conductivity of CeP2O7 for intermediate temperature fuel cells
journal, September 2008
- Sun, Xiufu; Wang, Shaorong; Wang, Zhenrong
- Solid State Ionics, Vol. 179, Issue 21-26
Solid acids as fuel cell electrolytes
journal, April 2001
- Haile, Sossina M.; Boysen, Dane A.; Chisholm, Calum R. I.
- Nature, Vol. 410, Issue 6831
Influence of phosphate anion adsorption on the kinetics of oxygen electroreduction on low index Pt(hkl) single crystals
journal, January 2010
- He, Qinggang; Yang, Xiaofang; Chen, Wei
- Physical Chemistry Chemical Physics, Vol. 12, Issue 39
Proton conductivity of mesoporous zirconium phosphate pyrophosphate
journal, October 1999
- Alberti, Giulio; Casciola, Mario; Cavalaglio, Silvia
- Solid State Ionics, Vol. 125, Issue 1-4
Efficient, Anhydrous Proton-Conducting Nanofilms of Y-Doped Zirconium Pyrophosphate at Intermediate Temperatures
journal, June 2008
- Li, Yuanzhi; Kunitake, Toyoki; Aoki, Yoshitaka
- Advanced Materials, Vol. 20, Issue 12
A Proton-Conducting In[sup 3+]-Doped SnP[sub 2]O[sub 7] Electrolyte for Intermediate-Temperature Fuel Cells
journal, January 2006
- Nagao, Masahiro; Takeuchi, Akihiko; Heo, Pilwon
- Electrochemical and Solid-State Letters, Vol. 9, Issue 3
Transformation of Rusty Stainless-Steel Meshes into Stable, Low-Cost, and Binder-Free Cathodes for High-Performance Potassium-Ion Batteries
journal, June 2017
- Zhu, Yun-hai; Yin, Yan-bin; Yang, Xu
- Angewandte Chemie, Vol. 129, Issue 27
Porous Hollow Carbon@Sulfur Composites for High-Power Lithium-Sulfur Batteries
journal, May 2011
- Jayaprakash, N.; Shen, J.; Moganty, Surya S.
- Angewandte Chemie International Edition, Vol. 50, Issue 26, p. 5904-5908
The Membrane–Electrode Interface in PEFCs
journal, January 2010
- Kim, Yu Seung; Pivovar, Bryan S.
- Journal of The Electrochemical Society, Vol. 157, Issue 11
High-Performance Solid Acid Fuel Cells Through Humidity Stabilization.
journal, April 2004
- Boysen, Dane A.; Uda, Tetsuya; Chisholm, Calum R. I.
- ChemInform, Vol. 35, Issue 14
The Membrane–Electrode Interface in PEFCs
journal, January 2010
- Kim, Yu Seung; Pivovar, Bryan S.
- Journal of The Electrochemical Society, Vol. 157, Issue 11
Works referencing / citing this record:
The Nature of Proton Shuttling in Protic Ionic Liquid Fuel Cells
journal, May 2019
- Smith, Daniel E.; Walsh, Darren A.
- Advanced Energy Materials, Vol. 9, Issue 24
Design of sepiolite-supported ionogel-embedded composite membranes without proton carrier wastage for wide-temperature-range operation of proton exchange membrane fuel cells
journal, January 2019
- Zhang, Xiaoxiao; Fu, Xudong; Yang, Shoukun
- Journal of Materials Chemistry A, Vol. 7, Issue 25
Figures / Tables found in this record: