Myceliophthora thermophila M77 utilizes hydrolytic and oxidative mechanisms to deconstruct biomass
Abstract
Biomass is abundant, renewable and useful for biofuel production as well as chemical priming for plastics and composites. Deconstruction of biomass by enzymes is perceived as recalcitrant while an inclusive breakdown mechanism remains to be discovered. Fungi such as Myceliophthora thermophila M77 appear to decompose natural biomass sources quite well. This work reports on this fungus fermentation property while producing cellulolytic enzymes using natural biomass substrates. Little hydrolytic activity was detected, insufficient to explain the large amount of biomass depleted in the process. Furthermore, this work makes a comprehensive account of extracellular proteins and describes how secretomes redirect their qualitative protein content based on the nature and chemistry of the nutritional source. Fungus grown on purified cellulose or on natural biomass produced secretomes constituted by: cellobiohydrolases, cellobiose dehydrogenase, β-1,3 glucanase, β-glucosidases, aldose epimerase, glyoxal oxidase, GH74 xyloglucanase, galactosidase, aldolactonase and polysaccharide monooxygenases. Fungus grown on a mixture of purified hemicellulose fractions (xylans, arabinans and arabinoxylans) produced many enzymes, some of which are listed here: xylosidase, mixed β-1,3(4) glucanase, β-1,3 glucanases, β-glucosidases, β-mannosidase, β-glucosidases, galactosidase, chitinases, polysaccharide lyase, endo β-1,6 galactanase and aldose epimerase. Secretomes produced on natural biomass displayed a comprehensive set of enzymes involved in hydrolysis and oxidation ofmore »
- Authors:
- Publication Date:
- Research Org.:
- Oklahoma State Univ., Stillwater, OK (United States)
- Sponsoring Org.:
- USDOE
- OSTI Identifier:
- 1330731
- Alternate Identifier(s):
- OSTI ID: 1375956
- Grant/Contract Number:
- 06103-OKL; ZDJ-7-77608-01; 403090/2012-1
- Resource Type:
- Journal Article: Published Article
- Journal Name:
- AMB Express
- Additional Journal Information:
- Journal Name: AMB Express Journal Volume: 6 Journal Issue: 1; Journal ID: ISSN 2191-0855
- Publisher:
- Springer Science + Business Media
- Country of Publication:
- Germany
- Language:
- English
- Subject:
- 09 BIOMASS FUELS; Myceliophthora thermophila; Biomass; Cellulose degradation; Secretome composition; Cellulose hydrolysis; Cellulose oxidation
Citation Formats
dos Santos, Hévila Brognaro, Bezerra, Thaís Milena Souza, Pradella, José G. C., Delabona, Priscila, Lima, Deise, Gomes, Eleni, Hartson, Steve D., Rogers, Janet, Couger, Brian, and Prade, Rolf. Myceliophthora thermophila M77 utilizes hydrolytic and oxidative mechanisms to deconstruct biomass. Germany: N. p., 2016.
Web. doi:10.1186/s13568-016-0276-y.
dos Santos, Hévila Brognaro, Bezerra, Thaís Milena Souza, Pradella, José G. C., Delabona, Priscila, Lima, Deise, Gomes, Eleni, Hartson, Steve D., Rogers, Janet, Couger, Brian, & Prade, Rolf. Myceliophthora thermophila M77 utilizes hydrolytic and oxidative mechanisms to deconstruct biomass. Germany. https://doi.org/10.1186/s13568-016-0276-y
dos Santos, Hévila Brognaro, Bezerra, Thaís Milena Souza, Pradella, José G. C., Delabona, Priscila, Lima, Deise, Gomes, Eleni, Hartson, Steve D., Rogers, Janet, Couger, Brian, and Prade, Rolf. 2016.
"Myceliophthora thermophila M77 utilizes hydrolytic and oxidative mechanisms to deconstruct biomass". Germany. https://doi.org/10.1186/s13568-016-0276-y.
@article{osti_1330731,
title = {Myceliophthora thermophila M77 utilizes hydrolytic and oxidative mechanisms to deconstruct biomass},
author = {dos Santos, Hévila Brognaro and Bezerra, Thaís Milena Souza and Pradella, José G. C. and Delabona, Priscila and Lima, Deise and Gomes, Eleni and Hartson, Steve D. and Rogers, Janet and Couger, Brian and Prade, Rolf},
abstractNote = {Biomass is abundant, renewable and useful for biofuel production as well as chemical priming for plastics and composites. Deconstruction of biomass by enzymes is perceived as recalcitrant while an inclusive breakdown mechanism remains to be discovered. Fungi such as Myceliophthora thermophila M77 appear to decompose natural biomass sources quite well. This work reports on this fungus fermentation property while producing cellulolytic enzymes using natural biomass substrates. Little hydrolytic activity was detected, insufficient to explain the large amount of biomass depleted in the process. Furthermore, this work makes a comprehensive account of extracellular proteins and describes how secretomes redirect their qualitative protein content based on the nature and chemistry of the nutritional source. Fungus grown on purified cellulose or on natural biomass produced secretomes constituted by: cellobiohydrolases, cellobiose dehydrogenase, β-1,3 glucanase, β-glucosidases, aldose epimerase, glyoxal oxidase, GH74 xyloglucanase, galactosidase, aldolactonase and polysaccharide monooxygenases. Fungus grown on a mixture of purified hemicellulose fractions (xylans, arabinans and arabinoxylans) produced many enzymes, some of which are listed here: xylosidase, mixed β-1,3(4) glucanase, β-1,3 glucanases, β-glucosidases, β-mannosidase, β-glucosidases, galactosidase, chitinases, polysaccharide lyase, endo β-1,6 galactanase and aldose epimerase. Secretomes produced on natural biomass displayed a comprehensive set of enzymes involved in hydrolysis and oxidation of cellulose, hemicellulose-pectin and lignin. Furthermore, the participation of oxidation reactions coupled to lignin decomposition in the breakdown of natural biomass may explain the discrepancy observed for cellulose decomposition in relation to natural biomass fermentation experiments.},
doi = {10.1186/s13568-016-0276-y},
url = {https://www.osti.gov/biblio/1330731},
journal = {AMB Express},
issn = {2191-0855},
number = 1,
volume = 6,
place = {Germany},
year = {Wed Nov 02 00:00:00 EDT 2016},
month = {Wed Nov 02 00:00:00 EDT 2016}
}
Web of Science
Figures / Tables:
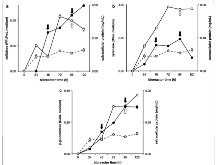 Fig. 1: Myceliophthora thermophila M77 fed-batch bioreactor with steam exploded sugar cane bagasse (SCBSE) as the carbon source. Bioreactors containing Mandels and Sternberg salts amended with 0.1% peptone and 1% steam exploded sugar cane bagasse (SCBSE) were conducted for 120 h at 45 °C, constant pH 5.0 and feed-pulsed withmore »
Fig. 1: Myceliophthora thermophila M77 fed-batch bioreactor with steam exploded sugar cane bagasse (SCBSE) as the carbon source. Bioreactors containing Mandels and Sternberg salts amended with 0.1% peptone and 1% steam exploded sugar cane bagasse (SCBSE) were conducted for 120 h at 45 °C, constant pH 5.0 and feed-pulsed withmore »
Works referenced in this record:
Phanerochaete chrysosporium produces a diverse array of extracellular enzymes when grown on sorghum
journal, January 2012
- Ray, Anamika; Saykhedkar, Sayali; Ayoubi-Canaan, Patricia
- Applied Microbiology and Biotechnology, Vol. 93, Issue 5
Novel enzymes for the degradation of cellulose
journal, January 2012
- Horn, Svein; Vaaje-Kolstad, Gustav; Westereng, Bjørge
- Biotechnology for Biofuels, Vol. 5, Issue 1, Article No. 45
Post-genomic insights into the plant polysaccharide degradation potential of Aspergillus nidulans and comparison to Aspergillus niger and Aspergillus oryzae
journal, March 2009
- Coutinho, Pedro M.; Andersen, Mikael R.; Kolenova, Katarina
- Fungal Genetics and Biology, Vol. 46, Issue 1
A comparative systems analysis of polysaccharide-elicited responses in Neurospora crassa reveals carbon source-specific cellular adaptations : Fungal polysaccharide-specific responses revealed
journal, December 2013
- Benz, J. Philipp; Chau, Bryant H.; Zheng, Diana
- Molecular Microbiology, Vol. 91, Issue 2
Lignocellulose conversion to biofuels: current challenges, global perspectives
journal, June 2009
- Himmel, Michael E.; Bayer, Edward A.
- Current Opinion in Biotechnology, Vol. 20, Issue 3
Assessment of the pectin degrading enzyme network of Aspergillus niger by functional genomics
journal, March 2009
- Martens-Uzunova, Elena S.; Schaap, Peter J.
- Fungal Genetics and Biology, Vol. 46, Issue 1
Purification and Properties of Cellobiose:Quinone Oxidoreductase from Sporotrichum pulverulentum.
journal, January 1975
- Westermark, Ulla; Eriksson, Karl-Erik; Hörnfeldt, A. -B.
- Acta Chemica Scandinavica, Vol. 29b
Selection of thermophilic and thermotolerant fungi for the production of cellulases and xylanases under solid-state fermentation
journal, January 2012
- Moretti, Marcia M. S.; Bocchini-Martins, Daniela A.; Silva, Roberto Da
- Brazilian Journal of Microbiology, Vol. 43, Issue 3
World crop residues production and implications of its use as a biofuel
journal, May 2005
- Lal, R.
- Environment International, Vol. 31, Issue 4
Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar
journal, March 1959
- Miller, G. L.
- Analytical Chemistry, Vol. 31, Issue 3
An Oxidative Enzyme Boosting the Enzymatic Conversion of Recalcitrant Polysaccharides
journal, October 2010
- Vaaje-Kolstad, Gustav; Westereng, Bjørge; Horn, Svein J.
- Science, Vol. 330, Issue 6001, p. 219-222
Increased enzymatic hydrolysis of sugarcane bagasse from enzyme recycling
journal, January 2015
- Visser, Evan; Leal, Tiago; de Almeida, Maíra
- Biotechnology for Biofuels, Vol. 8, Issue 1
A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding
journal, May 1976
- Bradford, Marion M.
- Analytical Biochemistry, Vol. 72, Issue 1-2
Milling pretreatment of sugarcane bagasse and straw for enzymatic hydrolysis and ethanol fermentation
journal, October 2010
- da Silva, Ayla Sant’Ana; Inoue, Hiroyuki; Endo, Takashi
- Bioresource Technology, Vol. 101, Issue 19
Is cellobiose dehydrogenase from Phanerochaete chrysosporium a lignin degrading enzyme?
journal, July 2000
- Henriksson, Gunnar; Zhang, Liming; Li, Jiebing
- Biochimica et Biophysica Acta (BBA) - Protein Structure and Molecular Enzymology, Vol. 1480, Issue 1-2
Mass Spectrometric Sequencing of Proteins from Silver-Stained Polyacrylamide Gels
journal, January 1996
- Shevchenko, Andrej; Wilm, Matthias; Vorm, Ole
- Analytical Chemistry, Vol. 68, Issue 5
Two structurally discrete GH7-cellobiohydrolases compete for the same cellulosic substrate fiber
journal, January 2012
- Segato, Fernando; Damasio, André R. L.; Gonçalves, Thiago
- Biotechnology for Biofuels, Vol. 5, Issue 1
Exponentially Modified Protein Abundance Index (emPAI) for Estimation of Absolute Protein Amount in Proteomics by the Number of Sequenced Peptides per Protein
journal, June 2005
- Ishihama, Yasushi; Oda, Yoshiya; Tabata, Tsuyoshi
- Molecular & Cellular Proteomics, Vol. 4, Issue 9
Insights into the oxidative degradation of cellulose by a copper metalloenzyme that exploits biomass components
journal, August 2011
- Quinlan, R. J.; Sweeney, M. D.; Lo Leggio, L.
- Proceedings of the National Academy of Sciences, Vol. 108, Issue 37, p. 15079-15084
Scientific challenges of bioethanol production in Brazil
journal, July 2011
- Amorim, Henrique V.; Lopes, Mário Lucio; de Castro Oliveira, Juliana Velasco
- Applied Microbiology and Biotechnology, Vol. 91, Issue 5
Transcriptome and exoproteome analysis of utilization of plant-derived biomass by Myceliophthora thermophila
journal, November 2014
- Kolbusz, Magdalena Anna; Di Falco, Marcos; Ishmael, Nadeeza
- Fungal Genetics and Biology, Vol. 72
Enzymatic hydrolysis of delignified bagasse polysaccharides
journal, October 2005
- Adsul, M. G.; Ghule, J. E.; Shaikh, H.
- Carbohydrate Polymers, Vol. 62, Issue 1
Activity Studies and Crystal Structures of Catalytically Deficient Mutants of Cellobiohydrolase I fromTrichoderma reesei
journal, November 1996
- Ståhlberg, Jerry; Divne, Christina; Koivula, Anu
- Journal of Molecular Biology, Vol. 264, Issue 2
Structural basis for cellobiose dehydrogenase action during oxidative cellulose degradation
journal, July 2015
- Tan, Tien-Chye; Kracher, Daniel; Gandini, Rosaria
- Nature Communications, Vol. 6, Issue 1
Cellobiose Dehydrogenase and a Copper-Dependent Polysaccharide Monooxygenase Potentiate Cellulose Degradation by Neurospora crassa
journal, December 2011
- Phillips, Christopher M.; Beeson, William T.; Cate, Jamie H.
- ACS Chemical Biology, Vol. 6, Issue 12, p. 1399-1406
Steam explosion pretreatment reproduction and alkaline delignification reactions performed on a pilot scale with sugarcane bagasse for bioethanol production
journal, January 2012
- Rocha, G. J. M.; Gonçalves, A. R.; Oliveira, B. R.
- Industrial Crops and Products, Vol. 35, Issue 1
Enzymatic hydrolysis of sugarcane bagasse pretreated with acid or alkali
journal, April 2011
- Pietrobon, Vivian Cristina; Monteiro, Regina Teresa Rosim; Pompeu, Georgia Bertoni
- Brazilian Archives of Biology and Technology, Vol. 54, Issue 2
Measurement of cellulase activities
journal, January 1987
- Ghose, T. K.
- Pure and Applied Chemistry, Vol. 59, Issue 2
Plant Cell Wall Deconstruction by Ascomycete Fungi
journal, September 2013
- Glass, N. Louise; Schmoll, Monika; Cate, Jamie H. D.
- Annual Review of Microbiology, Vol. 67, Issue 1
Comparative genomic analysis of the thermophilic biomass-degrading fungi Myceliophthora thermophila and Thielavia terrestris
journal, October 2011
- Berka, Randy M.; Grigoriev, Igor V.; Otillar, Robert
- Nature Biotechnology, Vol. 29, Issue 10, p. 922-927
Lignin Depletion Enhances the Digestibility of Cellulose in Cultured Xylem Cells
journal, July 2013
- Lacayo, Catherine I.; Hwang, Mona S.; Ding, Shi-You
- PLoS ONE, Vol. 8, Issue 7
Cellobiose Dehydrogenase – A Flavocytochrome from Wood-Degrading, Phytopathogenic and Saprotropic Fungi
journal, June 2006
- Zamocky, Marcel; Ludwig, R.; Peterbauer, C.
- Current Protein & Peptide Science, Vol. 7, Issue 3
The three-dimensional crystal structure of the catalytic core of cellobiohydrolase I from Trichoderma reesei
journal, July 1994
- Divne, C.; Stahlberg, J.; Reinikainen, T.
- Science, Vol. 265, Issue 5171
Cellobiose dehydrogenases of Sporotrichum (Chrysosporium) thermophile
journal, May 1991
- Canevascini, Giorgio; Borer, Peter; Dreyer, Jean-Luc
- European Journal of Biochemistry, Vol. 198, Issue 1
A time course analysis of the extracellular proteome of Aspergillus nidulans growing on sorghum stover
journal, January 2012
- Saykhedkar, Sayali; Ray, Anamika; Ayoubi-Canaan, Patricia
- Biotechnology for Biofuels, Vol. 5, Issue 1
Oxidative Cleavage of Cellulose by Fungal Copper-Dependent Polysaccharide Monooxygenases
journal, December 2011
- Beeson, William T.; Phillips, Christopher M.; Cate, Jamie H. D.
- Journal of the American Chemical Society, Vol. 134, Issue 2
Recent insights into copper-containing lytic polysaccharide mono-oxygenases
journal, October 2013
- Hemsworth, Glyn R.; Davies, Gideon J.; Walton, Paul H.
- Current Opinion in Structural Biology, Vol. 23, Issue 5, p. 660-668
Production of xylanolytic enzymes by strains of Aspergillus
journal, January 1989
- Bailey, MichaelJ.; Poutanen, Kaisa
- Applied Microbiology and Biotechnology, Vol. 30, Issue 1
Rotting by radicals – the role of cellobiose oxidoreductase?
journal, December 2003
- Mason, M. G.; Nicholls, P.; Wilson, M. T.
- Biochemical Society Transactions, Vol. 31, Issue 6
Works referencing / citing this record:
Biochemical and structural insights into a thermostable cellobiohydrolase from Myceliophthora thermophila
journal, December 2017
- Kadowaki, Marco A. S.; Higasi, Paula; Godoy, Mariana O.
- The FEBS Journal, Vol. 285, Issue 3
Oxidoreductases and Reactive Oxygen Species in Conversion of Lignocellulosic Biomass
journal, September 2018
- Bissaro, Bastien; Várnai, Anikó; Røhr, Åsmund K.
- Microbiology and Molecular Biology Reviews, Vol. 82, Issue 4
Transcriptome and secretome analysis of Aspergillus fumigatus in the presence of sugarcane bagasse
journal, April 2018
- de Gouvêa, Paula Fagundes; Bernardi, Aline Vianna; Gerolamo, Luis Eduardo
- BMC Genomics, Vol. 19, Issue 1
Functional characterization of a lytic polysaccharide monooxygenase from the thermophilic fungus Myceliophthora thermophila
journal, August 2018
- Kadowaki, Marco A. S.; Várnai, Anikó; Jameson, John-Kristian
- PLOS ONE, Vol. 13, Issue 8
Characterization of a New Glyoxal Oxidase from the Thermophilic Fungus Myceliophthora thermophila M77: Hydrogen Peroxide Production Retained in 5-Hydroxymethylfurfural Oxidation
journal, October 2018
- Kadowaki, Marco; Godoy, Mariana; Kumagai, Patricia
- Catalysts, Vol. 8, Issue 10
Figures / Tables found in this record: