Diastereo- and Enantioselective Iridium-Catalyzed Allylation of Cyclic Ketone Enolates: Synergetic Effect of Ligands and Barium Enolates
Journal Article
·
· Journal of the American Chemical Society
- Department of Chemistry, University of California, Berkeley, California 94720, United States
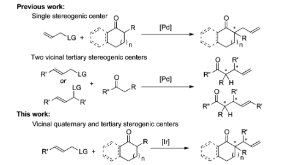
Here, we report asymmetric allylic alkylation of barium enolates of cyclic ketones catalyzed by a metallacyclic iridium complex containing a phosphoramidite ligand derived from (R)-1-(2-naphthyl)ethylamine. The reaction products contain adjacent quaternary and tertiary stereocenters. This process demonstrates that unstabilized cyclic ketone enolates can undergo diastereo- and enantioselective Ir-catalyzed allylic substitution reactions with the proper choice of enolate countercation. The products of these reactions can be conveniently transformed to various useful polycarbocyclic structures.
- Research Organization:
- Univ. of California, Berkeley, CA (United States)
- Sponsoring Organization:
- USDOE Office of Science (SC)
- Grant/Contract Number:
- AC02-05CH11231
- OSTI ID:
- 1163792
- Alternate ID(s):
- OSTI ID: 1345809
- Journal Information:
- Journal of the American Chemical Society, Journal Name: Journal of the American Chemical Society Vol. 136 Journal Issue: 45; ISSN 0002-7863
- Publisher:
- American Chemical SocietyCopyright Statement
- Country of Publication:
- United States
- Language:
- English
Cited by: 64 works
Citation information provided by
Web of Science
Web of Science
Similar Records
Enantioselective α-functionalizations of ketones via allylic substitution of silyl enol ethers
NHC-Catalyzed/Titanium(IV);#8722;Mediated Highly Diastereo- and Enantioselective Dimerization of Enals
Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism
Journal Article
·
Mon Nov 19 00:00:00 EST 2018
· Nature Chemistry
·
OSTI ID:1163792
NHC-Catalyzed/Titanium(IV);#8722;Mediated Highly Diastereo- and Enantioselective Dimerization of Enals
Journal Article
·
Wed May 09 00:00:00 EDT 2012
· Org. Lett.
·
OSTI ID:1163792
+2 more
Palladium/N-heterocyclic carbene catalysed regio and diastereoselective reaction of ketones with allyl reagents via inner-sphere mechanism
Journal Article
·
Fri Jun 10 00:00:00 EDT 2016
· Nature Communications
·
OSTI ID:1163792
+6 more