Low-Temperature Oxidation of Ethylene by Ozone in a Jet-Stirred Reactor
Abstract
Ethylene oxidation initiated by ozone addition (ozonolysis) is carried out in a jet-stirred reactor from 300-1000 K to explore the kinetic pathways relevant to low-temperature oxidation. The temperature dependencies of species’ mole fractions are quantified using molecular-beam mass spectrometry with electron ionization and single-photon ionization employing tunable synchrotron-generated vacuum-ultraviolet radiation. Upon ozone addition, significant ethylene oxidation is found in the low-temperature regime from 300-600 K. Here, we provide new insights into the ethylene ozonolysis reaction network via identification and quantification of previously elusive intermediates by combining experimental photoionization energy scans and ab initio threshold energy calculations for isomer identification. Specifically, the C2H4+O3 adduct C2H4O3 is identified as a keto-hydroperoxide (hydroperoxy-acetaldehyde, HOOCH2CHO) based on the calculated and experimentally observed ionization energy of 9.80 (±0.05) eV. Quantification using a photoionization cross-section of 5 Mb at 10.5 eV results in 5 ppm at atmospheric conditions which decreases monotonically with temperature until 550 K. Other hydroperoxide species, that contribute in larger amounts to the low-temperature oxidation of C2H4, like H2O2, CH3OOH, and C2H5OOH, are identified and their temperature-dependent mole fractions are reported. The experimental evidence for additional oxygenated species such as methanol, ketene, acetaldehyde, and hydroxy-acetaldehyde suggest multiple active oxidation routes. This experimental investigationmore »
- Authors:
-
- Princeton Univ., NJ (United States). Dept. of Mechanical and Aerospace Engineering
- Sandia National Lab. (SNL-CA), Livermore, CA (United States). Combustion Research Facility
- Argonne National Lab. (ANL), Argonne, IL (United States). Chemical Sciences and Engineering Division
- Publication Date:
- Research Org.:
- Argonne National Lab. (ANL), Argonne, IL (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); USDOE Office of Science (SC), Workforce Development for Teachers and Scientists (WDTS)
- OSTI Identifier:
- 1487124
- Grant/Contract Number:
- AC02-06CH11357
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Physical Chemistry. A, Molecules, Spectroscopy, Kinetics, Environment, and General Theory
- Additional Journal Information:
- Journal Volume: 122; Journal Issue: 43; Journal ID: ISSN 1089-5639
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; ethylene; jet-stirred reactor; keto-hydroperoxide; low-temperature chemistry; ozone
Citation Formats
Rousso, Aric C., Hansen, Nils, Jasper, Ahren W., and Ju, Yiguang. Low-Temperature Oxidation of Ethylene by Ozone in a Jet-Stirred Reactor. United States: N. p., 2018.
Web. doi:10.1021/acs.jpca.8b06556.
Rousso, Aric C., Hansen, Nils, Jasper, Ahren W., & Ju, Yiguang. Low-Temperature Oxidation of Ethylene by Ozone in a Jet-Stirred Reactor. United States. https://doi.org/10.1021/acs.jpca.8b06556
Rousso, Aric C., Hansen, Nils, Jasper, Ahren W., and Ju, Yiguang. Sun .
"Low-Temperature Oxidation of Ethylene by Ozone in a Jet-Stirred Reactor". United States. https://doi.org/10.1021/acs.jpca.8b06556. https://www.osti.gov/servlets/purl/1487124.
@article{osti_1487124,
title = {Low-Temperature Oxidation of Ethylene by Ozone in a Jet-Stirred Reactor},
author = {Rousso, Aric C. and Hansen, Nils and Jasper, Ahren W. and Ju, Yiguang},
abstractNote = {Ethylene oxidation initiated by ozone addition (ozonolysis) is carried out in a jet-stirred reactor from 300-1000 K to explore the kinetic pathways relevant to low-temperature oxidation. The temperature dependencies of species’ mole fractions are quantified using molecular-beam mass spectrometry with electron ionization and single-photon ionization employing tunable synchrotron-generated vacuum-ultraviolet radiation. Upon ozone addition, significant ethylene oxidation is found in the low-temperature regime from 300-600 K. Here, we provide new insights into the ethylene ozonolysis reaction network via identification and quantification of previously elusive intermediates by combining experimental photoionization energy scans and ab initio threshold energy calculations for isomer identification. Specifically, the C2H4+O3 adduct C2H4O3 is identified as a keto-hydroperoxide (hydroperoxy-acetaldehyde, HOOCH2CHO) based on the calculated and experimentally observed ionization energy of 9.80 (±0.05) eV. Quantification using a photoionization cross-section of 5 Mb at 10.5 eV results in 5 ppm at atmospheric conditions which decreases monotonically with temperature until 550 K. Other hydroperoxide species, that contribute in larger amounts to the low-temperature oxidation of C2H4, like H2O2, CH3OOH, and C2H5OOH, are identified and their temperature-dependent mole fractions are reported. The experimental evidence for additional oxygenated species such as methanol, ketene, acetaldehyde, and hydroxy-acetaldehyde suggest multiple active oxidation routes. This experimental investigation closes the gap between ozonolysis at atmospheric and elevated temperature conditions and provides a database for future modeling.},
doi = {10.1021/acs.jpca.8b06556},
journal = {Journal of Physical Chemistry. A, Molecules, Spectroscopy, Kinetics, Environment, and General Theory},
number = 43,
volume = 122,
place = {United States},
year = {Sun Oct 07 00:00:00 EDT 2018},
month = {Sun Oct 07 00:00:00 EDT 2018}
}
Web of Science
Figures / Tables:
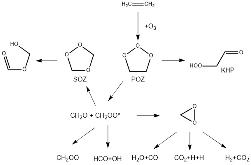 Figure 1: Ethylene ozonolysis reaction pathway with major intermediate species.
Figure 1: Ethylene ozonolysis reaction pathway with major intermediate species.
Works referenced in this record:
C.—The oxidation of hydrocarbons by ozone at low temperatures
journal, January 1906
- Drugman, Julien
- J. Chem. Soc., Trans., Vol. 89, Issue 0
Measurement and modelling of air pollution and atmospheric chemistry in the U.K. West Midlands conurbation: Overview of the PUMA Consortium project
journal, May 2006
- Harrison, R. M.; Yin, J.; Tilling, R. M.
- Science of The Total Environment, Vol. 360, Issue 1-3
Energetics, Kinetics, and Product Distributions of the Reactions of Ozone with Ethene and 2,3-Dimethyl-2-butene
journal, December 1997
- Olzmann, M.; Kraka, E.; Cremer, D.
- The Journal of Physical Chemistry A, Vol. 101, Issue 49
Highly Oxygenated Molecules from Atmospheric Autoxidation of Hydrocarbons: A Prominent Challenge for Chemical Kinetics Studies: HIGHLY OXYGENATED MOLECULES FROM ATMOSPHERIC AUTOXIDATION OF HYDROCARBONS
journal, September 2017
- Ehn, Mikael; Berndt, Torsten; Wildt, Jürgen
- International Journal of Chemical Kinetics, Vol. 49, Issue 11
The Formation of Highly Oxidized Multifunctional Products in the Ozonolysis of Cyclohexene
journal, October 2014
- Rissanen, Matti P.; Kurtén, Theo; Sipilä, Mikko
- Journal of the American Chemical Society, Vol. 136, Issue 44
Observation of the simplest Criegee intermediate CH 2 OO in the gas-phase ozonolysis of ethylene
journal, March 2015
- Womack, Caroline C.; Martin-Drumel, Marie-Aline; Brown, Gordon G.
- Science Advances, Vol. 1, Issue 2
Oligomerization Reaction of the Criegee Intermediate Leads to Secondary Organic Aerosol Formation in Ethylene Ozonolysis
journal, November 2013
- Sakamoto, Yosuke; Inomata, Satoshi; Hirokawa, Jun
- The Journal of Physical Chemistry A, Vol. 117, Issue 48
Water vapour effects on secondary organic aerosol formation in isoprene ozonolysis
journal, January 2017
- Sakamoto, Yosuke; Yajima, Ryoji; Inomata, Satoshi
- Physical Chemistry Chemical Physics, Vol. 19, Issue 4
The physical chemistry of Criegee intermediates in the gas phase
journal, July 2015
- Osborn, David L.; Taatjes, Craig A.
- International Reviews in Physical Chemistry, Vol. 34, Issue 3
Criegee Intermediates: What Direct Production and Detection Can Teach Us About Reactions of Carbonyl Oxides
journal, May 2017
- Taatjes, Craig A.
- Annual Review of Physical Chemistry, Vol. 68, Issue 1
Die Ozonisierung des 9,10-Oktalins
journal, July 1949
- Criegee, Rudolf; Wenner, Gotthilf
- Justus Liebigs Annalen der Chemie, Vol. 564, Issue 1
Quantum Chemical Study of the Initial Step of Ozone Addition to the Double Bond of Ethylene
journal, October 2012
- Gadzhiev, Oleg B.; Ignatov, Stanislav K.; Krisyuk, Boris E.
- The Journal of Physical Chemistry A, Vol. 116, Issue 42
Kinetics of CH 2 OO reactions with SO 2 , NO 2 , NO, H 2 O and CH 3 CHO as a function of pressure
journal, January 2014
- Stone, Daniel; Blitz, Mark; Daubney, Laura
- Phys. Chem. Chem. Phys., Vol. 16, Issue 3
The reactions of Criegee intermediates with alkenes, ozone, and carbonyl oxides
journal, January 2014
- Vereecken, L.; Harder, H.; Novelli, A.
- Physical Chemistry Chemical Physics, Vol. 16, Issue 9
Nascent energy distribution of the Criegee intermediate CH 2 OO from direct dynamics calculations of primary ozonide dissociation
journal, May 2018
- Pfeifle, Mark; Ma, Yong-Tao; Jasper, Ahren W.
- The Journal of Chemical Physics, Vol. 148, Issue 17
Stabilization of the Simplest Criegee Intermediate from the Reaction between Ozone and Ethylene: A High-Level Quantum Chemical and Kinetic Analysis of Ozonolysis
journal, May 2015
- Nguyen, Thanh Lam; Lee, Hyunwoo; Matthews, Devin A.
- The Journal of Physical Chemistry A, Vol. 119, Issue 22
Identification of dioxirane (H2COO) in ozone-olefin reactions via microwave spectroscopy
journal, November 1977
- Lovas, F. J.; Suenram, R. D.
- Chemical Physics Letters, Vol. 51, Issue 3
Formation of hydroxymethyl hydroperoxide and formic acid in alkene ozonolysis in the presence of water vapour
journal, May 1997
- Neeb, Peter; Sauer, Frank; Horie, Osamu
- Atmospheric Environment, Vol. 31, Issue 10
First-principles-derived kinetics of the reactions involved in low-temperature dimethyl ether oxidation
journal, February 2008
- Andersen, Amity; Carter, Emily A.
- Molecular Physics, Vol. 106, Issue 2-4
Plasma assisted combustion: Dynamics and chemistry
journal, June 2015
- Ju, Yiguang; Sun, Wenting
- Progress in Energy and Combustion Science, Vol. 48
The effect of ozone addition on laminar flame speed
journal, October 2015
- Gao, Xiang; Zhang, Yao; Adusumilli, Sampath
- Combustion and Flame, Vol. 162, Issue 10
Kinetic studies of ozone assisted low temperature oxidation of dimethyl ether in a flow reactor using molecular-beam mass spectrometry
journal, November 2016
- Zhao, Hao; Yang, Xueliang; Ju, Yiguang
- Combustion and Flame, Vol. 173
Spark ignition of methane and methanol in ozonized air
journal, January 1989
- Nomaguchi, T.; Koda, S.
- Symposium (International) on Combustion, Vol. 22, Issue 1
Flame propagation enhancement by plasma excitation of oxygen. Part I: Effects of O3
journal, October 2010
- Ombrello, Timothy; Won, Sang Hee; Ju, Yiguang
- Combustion and Flame, Vol. 157, Issue 10
Quantification of the Keto-Hydroperoxide (HOOCH 2 OCHO) and Other Elusive Intermediates during Low-Temperature Oxidation of Dimethyl Ether
journal, September 2016
- Moshammer, Kai; Jasper, Ahren W.; Popolan-Vaida, Denisia M.
- The Journal of Physical Chemistry A, Vol. 120, Issue 40
Detection and Identification of the Keto-Hydroperoxide (HOOCH 2 OCHO) and Other Intermediates during Low-Temperature Oxidation of Dimethyl Ether
journal, February 2015
- Moshammer, Kai; Jasper, Ahren W.; Popolan-Vaida, Denisia M.
- The Journal of Physical Chemistry A, Vol. 119, Issue 28
New insights into the low-temperature oxidation of 2-methylhexane
journal, January 2017
- Wang, Zhandong; Mohamed, Samah Y.; Zhang, Lidong
- Proceedings of the Combustion Institute, Vol. 36, Issue 1
Absorption cross-sections of ozone in the ultraviolet and visible spectral regions: Status report 2015
journal, September 2016
- Orphal, Johannes; Staehelin, Johannes; Tamminen, Johanna
- Journal of Molecular Spectroscopy, Vol. 327
Kinetic modeling of ethylene oxidation
journal, March 1988
- Dagaut, P.
- Combustion and Flame, Vol. 71, Issue 3
Chemical dynamics, molecular energetics, and kinetics at the synchrotron
journal, January 2010
- Leone, Stephen R.; Ahmed, Musahid; Wilson, Kevin R.
- Physical Chemistry Chemical Physics, Vol. 12, Issue 25
Advances and challenges in laminar flame experiments and implications for combustion chemistry
journal, August 2014
- Egolfopoulos, F. N.; Hansen, N.; Ju, Y.
- Progress in Energy and Combustion Science, Vol. 43
Detailed mass spectrometric and modeling study of isomeric butene flames
journal, March 2013
- Schenk, Marina; Leon, Larisa; Moshammer, Kai
- Combustion and Flame, Vol. 160, Issue 3
Near-threshold absolute photoionization cross-sections of some reaction intermediates in combustion
journal, February 2008
- Wang, Juan; Yang, Bin; Cool, Terrill A.
- International Journal of Mass Spectrometry, Vol. 269, Issue 3
Comprehensive kinetic modeling and experimental study of a fuel-rich, premixed n-heptane flame
journal, May 2015
- Seidel, Lars; Moshammer, Kai; Wang, Xiaoxiao
- Combustion and Flame, Vol. 162, Issue 5
Photoionization mass spectrometer for studies of flame chemistry with a synchrotron light source
journal, September 2005
- Cool, Terrill A.; McIlroy, Andrew; Qi, Fei
- Review of Scientific Instruments, Vol. 76, Issue 9
Selective detection of isomers with photoionization mass spectrometry for studies of hydrocarbon flame chemistry
journal, October 2003
- Cool, Terrill A.; Nakajima, Koichi; Mostefaoui, Toufik A.
- The Journal of Chemical Physics, Vol. 119, Issue 16
Recent contributions of flame-sampling molecular-beam mass spectrometry to a fundamental understanding of combustion chemistry
journal, April 2009
- Hansen, Nils; Cool, Terrill A.; Westmoreland, Phillip R.
- Progress in Energy and Combustion Science, Vol. 35, Issue 2
“Imaging” combustion chemistry via multiplexed synchrotron-photoionization mass spectrometry
journal, January 2008
- Taatjes, Craig A.; Hansen, Nils; Osborn, David L.
- Phys. Chem. Chem. Phys., Vol. 10, Issue 1
Studies of a fuel-rich propane flame with photoionization mass spectrometry
journal, January 2005
- Cool, Terrill A.; Nakajima, Koichi; Taatjes, Craig A.
- Proceedings of the Combustion Institute, Vol. 30, Issue 1
Ethylene pyrolysis and oxidation: A kinetic modeling study
journal, June 1990
- Dagaut, Philippe; Boettner, Jean-Claude; Cathonnet, Michel
- International Journal of Chemical Kinetics, Vol. 22, Issue 6
Ethylene oxidation in a well-stirred reactor
journal, October 1995
- Marinov, Nick M.; Malte, Philip C.
- International Journal of Chemical Kinetics, Vol. 27, Issue 10
The oxidation of 2-butene: A high pressure ignition delay, kinetic modeling study and reactivity comparison with isobutene and 1-butene
journal, January 2017
- Li, Yang; Zhou, Chong-Wen; Somers, Kieran P.
- Proceedings of the Combustion Institute, Vol. 36, Issue 1
A Hierarchical and Comparative Kinetic Modeling Study of C 1 − C 2 Hydrocarbon and Oxygenated Fuels : KINETIC STUDY OF C
journal, August 2013
- Metcalfe, Wayne K.; Burke, Sinéad M.; Ahmed, Syed S.
- International Journal of Chemical Kinetics, Vol. 45, Issue 10
Effect of prompt dissociation of formyl radical on 1,3,5-trioxane and CH2O laminar flame speeds with CO2 dilution at elevated pressure
journal, September 2017
- Zhao, Hao; Fu, Jiapeng; Haas, Francis M.
- Combustion and Flame, Vol. 183
An Experimental and Kinetic Study of Ethene Oxidation at a High Equivalence Ratio
journal, October 2002
- Jallais, S.; Bonneau, L.; Auzanneau, M.
- Industrial & Engineering Chemistry Research, Vol. 41, Issue 23
Experimental Confirmation of the Low-Temperature Oxidation Scheme of Alkanes
journal, April 2010
- Battin-Leclerc, Frédérique; Herbinet, Olivier; Glaude, Pierre-Alexandre
- Angewandte Chemie International Edition, Vol. 49, Issue 18
The nature of the transitory product in the gas-phase ozonolysis of ethene
journal, November 1995
- Neeb, Peter; Horie, Osamu; Moortgat, Geert K.
- Chemical Physics Letters, Vol. 246, Issue 1-2
Synchrotron photoionization mass spectrometry study of intermediates in fuel-rich 1,2-dimethoxyethane flame
journal, April 2009
- Lin, Z. K.; Han, D. L.; Li, S. F.
- The Journal of Chemical Physics, Vol. 130, Issue 15
Direct Kinetic Measurements of Reactions between the Simplest Criegee Intermediate CH 2 OO and Alkenes
journal, March 2014
- Buras, Zachary J.; Elsamra, Rehab M. I.; Jalan, Amrit
- The Journal of Physical Chemistry A, Vol. 118, Issue 11
Plasma supported combustion
journal, January 2005
- Starikovskii, A. Yu.
- Proceedings of the Combustion Institute, Vol. 30, Issue 2
Effect of ozone on combustion of compression ignition engines
journal, June 1991
- Tachibana, T.; Hirata, K.; Nishida, H.
- Combustion and Flame, Vol. 85, Issue 3-4
Direct measurement of Criegee intermediate (CH2OO) reactions with acetone, acetaldehyde, and hexafluoroacetone
journal, January 2012
- Taatjes, Craig A.; Welz, Oliver; Eskola, Arkke J.
- Physical Chemistry Chemical Physics, Vol. 14, Issue 30
Formation of formic acid and organic peroxides in the ozonolysis of ethene with added water vapour
journal, July 1994
- Horie, Osamu; Neeb, Peter; Limbach, Stefan
- Geophysical Research Letters, Vol. 21, Issue 14
VUV Photoionization Cross Sections of HO 2 , H 2 O 2 , and H 2 CO
journal, February 2015
- Dodson, Leah G.; Shen, Linhan; Savee, John D.
- The Journal of Physical Chemistry A, Vol. 119, Issue 8
Photolysis of methane revisited at 121.6 nm and at 118.2 nm: quantum yields of the primary products, measured by mass spectrometry
journal, January 2011
- Gans, Bérenger; Boyé-Péronne, Séverine; Broquier, Michel
- Physical Chemistry Chemical Physics, Vol. 13, Issue 18
Calculation of low‐energy elastic cross sections for electron‐CF 4 scattering
journal, May 1994
- Gianturco, F. A.; Lucchese, R. R.; Sanna, N.
- The Journal of Chemical Physics, Vol. 100, Issue 9
A Near-Threshold Shape Resonance in the Valence-Shell Photoabsorption of Linear Alkynes
journal, October 2015
- Jacovella, U.; Holland, D. M. P.; Boyé-Péronne, S.
- The Journal of Physical Chemistry A, Vol. 119, Issue 50
High-resolution vacuum-ultraviolet photoabsorption spectra of 1-butyne and 2-butyne
journal, July 2015
- Jacovella, U.; Holland, D. M. P.; Boyé-Péronne, S.
- The Journal of Chemical Physics, Vol. 143, Issue 3
Cross section and asymmetry parameter calculation for sulfur 1s photoionization of SF6
journal, September 1999
- Natalense, Alexandra P. P.; Lucchese, Robert R.
- The Journal of Chemical Physics, Vol. 111, Issue 12
Developing detailed chemical kinetic mechanisms for fuel combustion
journal, January 2019
- Curran, Henry J.
- Proceedings of the Combustion Institute, Vol. 37, Issue 1
Photoionization cross sections for reaction intermediates in hydrocarbon combustion
journal, December 2005
- Cool, Terrill A.; Wang, Juan; Nakajima, Koichi
- International Journal of Mass Spectrometry, Vol. 247, Issue 1-3
Absolute photoionization cross-sections of some combustion intermediates
journal, January 2012
- Yang, Bin; Wang, Juan; Cool, Terrill A.
- International Journal of Mass Spectrometry, Vol. 309
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal