Rates of Ligand Exchange around the Bis-Oxalato Complex [NpO2(C2O4)2]3- Measured by Using Multinuclear NMR Spectroscopy under Neutral to Semi-Alkaline Conditions [Rates of ligand exchange around the bis-oxalato complex NpO2(C2O4)23- measured using multinuclear NMR at neutral to semi-alkaline conditions]
Abstract
The kinetics of ligand exchange between the free oxalate ion, C2O42-, and the bis-oxalato NpV complex, [NpO2(C2O4)2]3-, in aqueous solution are reported by using 13C and 17O NMR spectroscopy methods. Rates of exchange were measured in the pH regime of 6.5–9.0, at which speciation is shown to be suitably simple. Because the neptunium(V) complex is paramagnetic, the rates of ligand exchange were estimated by following the width of the 13C and 17O signals assigned to the free oxalate ion in solution and by applying the Swift–Connick method for measuring rates of exchange. A set of experiments were conducted in which pH and total oxalate concentration were varied, and the linear dependence of the rate on these parameters was demonstrated. Variable-temperature NMR spectroscopy was also performed to measure activation parameters of complexation. Finally, at pH<8.0, ΔH≠=16.9 ±4.9 kJ mol-1 and ΔS≠=-116.3 ±17.1kJ mol-1K-1, whereas at pH>8.0 there is almost no dependence on temperature, which is interpreted to indicate that hydrolysis is coupled to ligand exchange under these conditions.
- Authors:
-
- Univ. of California, Davis, CA (United States). Dept. of Chemistry
- Lawrence Livermore National Lab. (LLNL), Livermore, CA (United States)
- Publication Date:
- Research Org.:
- Lawrence Livermore National Laboratory (LLNL), Livermore, CA (United States); Energy Frontier Research Centers (EFRC) (United States). Materials Science of Actinides (MSA)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); USDOE Office of Science (SC), Biological and Environmental Research (BER)
- OSTI Identifier:
- 1466938
- Alternate Identifier(s):
- OSTI ID: 1423490
- Report Number(s):
- LLNL-JRNL-744744
Journal ID: ISSN 2192-6506; 899908
- Grant/Contract Number:
- AC52-07NA27344; SC0001089
- Resource Type:
- Accepted Manuscript
- Journal Name:
- ChemPlusChem
- Additional Journal Information:
- Journal Volume: 83; Journal Issue: 7; Journal ID: ISSN 2192-6506
- Publisher:
- ChemPubSoc Europe
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; kinetics; ligand effects; neptunium; NMR spectroscopy; radiochemistry
Citation Formats
Pilgrim, Corey D., Mason, Harris E., Zavarin, Mavrik, and Casey, William H. Rates of Ligand Exchange around the Bis-Oxalato Complex [NpO2(C2O4)2]3- Measured by Using Multinuclear NMR Spectroscopy under Neutral to Semi-Alkaline Conditions [Rates of ligand exchange around the bis-oxalato complex NpO2(C2O4)23- measured using multinuclear NMR at neutral to semi-alkaline conditions]. United States: N. p., 2018.
Web. doi:10.1002/cplu.201800025.
Pilgrim, Corey D., Mason, Harris E., Zavarin, Mavrik, & Casey, William H. Rates of Ligand Exchange around the Bis-Oxalato Complex [NpO2(C2O4)2]3- Measured by Using Multinuclear NMR Spectroscopy under Neutral to Semi-Alkaline Conditions [Rates of ligand exchange around the bis-oxalato complex NpO2(C2O4)23- measured using multinuclear NMR at neutral to semi-alkaline conditions]. United States. https://doi.org/10.1002/cplu.201800025
Pilgrim, Corey D., Mason, Harris E., Zavarin, Mavrik, and Casey, William H. Fri .
"Rates of Ligand Exchange around the Bis-Oxalato Complex [NpO2(C2O4)2]3- Measured by Using Multinuclear NMR Spectroscopy under Neutral to Semi-Alkaline Conditions [Rates of ligand exchange around the bis-oxalato complex NpO2(C2O4)23- measured using multinuclear NMR at neutral to semi-alkaline conditions]". United States. https://doi.org/10.1002/cplu.201800025. https://www.osti.gov/servlets/purl/1466938.
@article{osti_1466938,
title = {Rates of Ligand Exchange around the Bis-Oxalato Complex [NpO2(C2O4)2]3- Measured by Using Multinuclear NMR Spectroscopy under Neutral to Semi-Alkaline Conditions [Rates of ligand exchange around the bis-oxalato complex NpO2(C2O4)23- measured using multinuclear NMR at neutral to semi-alkaline conditions]},
author = {Pilgrim, Corey D. and Mason, Harris E. and Zavarin, Mavrik and Casey, William H.},
abstractNote = {The kinetics of ligand exchange between the free oxalate ion, C2O42-, and the bis-oxalato NpV complex, [NpO2(C2O4)2]3-, in aqueous solution are reported by using 13C and 17O NMR spectroscopy methods. Rates of exchange were measured in the pH regime of 6.5–9.0, at which speciation is shown to be suitably simple. Because the neptunium(V) complex is paramagnetic, the rates of ligand exchange were estimated by following the width of the 13C and 17O signals assigned to the free oxalate ion in solution and by applying the Swift–Connick method for measuring rates of exchange. A set of experiments were conducted in which pH and total oxalate concentration were varied, and the linear dependence of the rate on these parameters was demonstrated. Variable-temperature NMR spectroscopy was also performed to measure activation parameters of complexation. Finally, at pH<8.0, ΔH≠=16.9 ±4.9 kJ mol-1 and ΔS≠=-116.3 ±17.1kJ mol-1K-1, whereas at pH>8.0 there is almost no dependence on temperature, which is interpreted to indicate that hydrolysis is coupled to ligand exchange under these conditions.},
doi = {10.1002/cplu.201800025},
journal = {ChemPlusChem},
number = 7,
volume = 83,
place = {United States},
year = {Fri Mar 02 00:00:00 EST 2018},
month = {Fri Mar 02 00:00:00 EST 2018}
}
Figures / Tables:
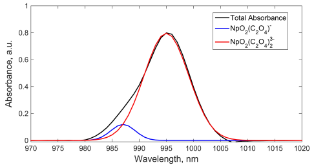 Figure 1: A representative absorbance spectrum of the mixture of NpO2(C2O4)- and NpO2(C2O4)3- in aqueous solution, with no free NpO2+ seen.
Figure 1: A representative absorbance spectrum of the mixture of NpO2(C2O4)- and NpO2(C2O4)3- in aqueous solution, with no free NpO2+ seen.
Works referenced in this record:
Calcium Oxalate: Occurrence in Soils and Effect on Nutrient and Geochemical Cycles
journal, December 1977
- Graustein, W. C.; Cromack, K.; Sollins, P.
- Science, Vol. 198, Issue 4323
Structural Variability in Neptunium(V) Oxalate Compounds: Synthesis and Structural Characterization of Na 2 NpO 2 (C 2 O 4 )OH·H 2 O
journal, October 2004
- Bean, Amanda C.; Garcia, Eduardo; Scott, Brian L.
- Inorganic Chemistry, Vol. 43, Issue 20
The specific activity and half-life of 237Np
journal, February 1960
- Brauer, F. P.; Stromatt, R. W.; Ludwick, J. D.
- Journal of Inorganic and Nuclear Chemistry, Vol. 12, Issue 3-4
Some applications of the transition state method to the calculation of reaction velocities, especially in solution
journal, January 1935
- Evans, M. G.; Polanyi, M.
- Transactions of the Faraday Society, Vol. 31
Equilibria and dynamics in binary and ternary uranyl oxalate and acetate/fluoride complexes †
journal, January 1999
- Aas, Wenche; Szabó, Zoltán; Grenthe, Ingmar
- Journal of the Chemical Society, Dalton Transactions, Issue 8
Quantum Chemical Study of the Water Exchange Mechanism of the Americyl(VI) Aqua Ion
journal, October 2016
- Fabrizio, Alberto; Rotzinger, François P.
- Inorganic Chemistry, Vol. 55, Issue 21
880. The reactivity of co-ordinated oxalated. Part I. Oxygen-18 exchange studies on oxalic acid, the trisoxalatochromium(III) anion, and the trisoxalatocobalt(III) anion
journal, January 1964
- Bunton, C. A.; Carter, J. H.; Llewellyn, D. R.
- Journal of the Chemical Society (Resumed)
Radioactive Element 93
journal, June 1940
- McMillan, Edwin; Abelson, Philip Hauge
- Physical Review, Vol. 57, Issue 12
Kinetic Studies of the [NpO 2 (CO 3 ) 3 ] 4– Ion at Alkaline Conditions Using 13 C NMR
journal, March 2014
- Panasci, Adele F.; Harley, Stephen J.; Zavarin, Mavrik
- Inorganic Chemistry, Vol. 53, Issue 8
Pressure Dependence of Carbonate Exchange with [NpO 2 (CO 3 ) 3 ] 4– in Aqueous Solutions
journal, December 2016
- Pilgrim, Corey D.; Zavarin, Mavrik; Casey, William H.
- Inorganic Chemistry, Vol. 56, Issue 1
NMR‐Relaxation Mechanisms of O 17 in Aqueous Solutions of Paramagnetic Cations and the Lifetime of Water Molecules in the First Coordination Sphere
journal, July 1962
- Swift, T. J.; Connick, Robert E.
- The Journal of Chemical Physics, Vol. 37, Issue 2
Spectrophotometric Study of Np(V) Oxalate Complexes 1
journal, August 1953
- Gruen, D. M.; Katz, J. J.
- Journal of the American Chemical Society, Vol. 75, Issue 15
Reaction mechanisms of the thermal conversion of Pu(IV) oxalate into plutonium oxide
journal, October 2007
- Vigier, N.; Grandjean, S.; Arab-Chapelet, B.
- Journal of Alloys and Compounds, Vol. 444-445
Actinides in Solution: Complexation and Kinetics
book, January 2010
- Choppin, Gregory R.; Jensen, Mark P.
- The Chemistry of the Actinide and Transactinide Elements
The Activated Complex in Chemical Reactions
journal, February 1935
- Eyring, Henry
- The Journal of Chemical Physics, Vol. 3, Issue 2
Crystal Structure of a Double Oxalate of Np(V) and Co(NH3) 6 3+ , Co(NH3)6NpO2(C2O4)2·1.5H2O
journal, November 2005
- Charushnikova, I. A.; Krot, N. N.; Polyakova, I. N.
- Radiochemistry, Vol. 47, Issue 6
Analysis of trace neptunium in the vicinity of underground nuclear tests at the Nevada National Security Site
journal, November 2014
- Zhao, P.; Tinnacher, R. M.; Zavarin, M.
- Journal of Environmental Radioactivity, Vol. 137
Inorganic and Bioinorganic Solvent Exchange Mechanisms
journal, June 2005
- Helm, Lothar; Merbach, André E.
- Chemical Reviews, Vol. 105, Issue 6
Carbonate exchange for the complex tris(carbonato)dioxouranate(4-) in aqueous solution as studied by carbon-13-NMR spectroscopy
journal, May 1991
- Brucher, Erno.; Glaser, Julius.; Toth, Imre.
- Inorganic Chemistry, Vol. 30, Issue 9
Relaxation Effects in Nuclear Magnetic Resonance Absorption
journal, April 1948
- Bloembergen, N.; Purcell, E. M.; Pound, R. V.
- Physical Review, Vol. 73, Issue 7
Plutonium Transport in the Environment
journal, June 2012
- Kersting, Annie B.
- Inorganic Chemistry, Vol. 52, Issue 7
The Rates and Mechanisms of Water Exchange of Actinide Aqua Ions: A Variable Temperature 17 O NMR Study of U(H 2 O) 10 4+ , UF(H 2 O) 9 3+ , and Th(H 2 O) 10 4+
journal, February 2000
- Farkas, Ildikó; Grenthe, Ingmar; Bányai, István
- The Journal of Physical Chemistry A, Vol. 104, Issue 6
Actinide speciation in aquatic systems
journal, March 2006
- Choppin, Gregory R.
- Marine Chemistry, Vol. 99, Issue 1-4
Kinetics of Ligand Exchange Reactions for Uranyl(2+) Fluoride Complexes in Aqueous Solution
journal, January 1996
- Szabó, Zoltán; Glaser, Julius; Grenthe, Ingmar
- Inorganic Chemistry, Vol. 35, Issue 7
Multinuclear NMR, Raman, EXAFS, and X-ray diffraction studies of uranyl carbonate complexes in near-neutral aqueous solution. X-ray structure of [C(NH2)3]6[(UO2)3(CO3)6].cntdot.6.5H2O
journal, September 1995
- Allen, P. G.; Bucher, J. J.; Clark, D. L.
- Inorganic Chemistry, Vol. 34, Issue 19
Geochemical kinetics via the Swift–Connick equations and solution NMR
journal, July 2011
- Harley, Steven J.; Ohlin, C. André; Casey, William H.
- Geochimica et Cosmochimica Acta, Vol. 75, Issue 13
13C NMR characterization of actinyl(VI) carbonate complexes in aqueous solution
journal, March 1993
- Clark, David L.; Hobart, David E.; Palmer, Phillip D.
- Journal of Alloys and Compounds, Vol. 193, Issue 1-2
Rates and Mechanisms of Water Exchange of UO 2 2+ (aq) and UO 2 (oxalate)F(H 2 O) 2 - : A Variable-Temperature 17 O and 19 F NMR Study
journal, February 2000
- Farkas, Ildikó; Bányai, István; Szabó, Zoltán
- Inorganic Chemistry, Vol. 39, Issue 4
The Sulfate Coordination of Np(IV), Np(V), and Np(VI) in Aqueous Solution
journal, June 2009
- Hennig, Christoph; Ikeda-Ohno, Atsushi; Tsushima, Satoru
- Inorganic Chemistry, Vol. 48, Issue 12
Investigation of Aquo and Chloro Complexes of UO 2 2+ , NpO 2 + , Np 4+ , and Pu 3+ by X-ray Absorption Fine Structure Spectroscopy
journal, October 1997
- Allen, P. G.; Bucher, J. J.; Shuh, D. K.
- Inorganic Chemistry, Vol. 36, Issue 21
Water exchange on metal ions: experiments and simulations
journal, June 1999
- Helm, L.; Merbach, A. E.
- Coordination Chemistry Reviews, Vol. 187, Issue 1
Symmetry, Optical Properties and Thermodynamics of Neptunium(V) Complexes
journal, December 2009
- Rao, Linfeng; Tian, Guoxin
- Symmetry, Vol. 2, Issue 1
Structure and Bonding in Solution of Dioxouranium(VI) Oxalate Complexes: Isomers and Intramolecular Ligand Exchange
journal, March 2003
- Vallet, Valérie; Moll, Henry; Wahlgren, Ulf
- Inorganic Chemistry, Vol. 42, Issue 6
Inorganic and Bioinorganic Solvent Exchange Mechanisms
journal, September 2005
- Helm, Lothar; Merbach, Andre E.
- ChemInform, Vol. 36, Issue 37
Structure and Bonding in Solution of Dioxouranium(VI) Oxalate Complexes: Isomers and Intramolecular Ligand Exchange
journal, December 2003
- Vallet, Valérie; Moll, Henry; Wahlgren, Ulf
- Inorganic Chemistry, Vol. 42, Issue 25
Relaxation Effects in Nuclear Magnetic Resonance Absorption
conference, July 2014
- Bloembergen, N.; Purcell, E. M.; Pound, R. V.
- A Volume in Honor of the 70th Birthday of Nicolaas Bloembergen, Resonances

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal