Evidence for distinct rate-limiting steps in the cleavage of alkenes by carotenoid cleavage dioxygenases
Abstract
Carotenoid cleavage dioxygenases (CCDs) use a nonheme Fe(II) cofactor to split alkene bonds of carotenoid and stilbenoid substrates. The iron centers of CCDs are typically five-coordinate in their resting states, with solvent occupying an exchangeable site. The involvement of this iron-bound solvent in CCD catalysis has not been experimentally addressed, but computational studies suggest two possible roles. 1) Solvent dissociation provides a coordination site for O2, or 2) solvent remains bound to iron but changes its equilibrium position to allow O2 binding and potentially acts as a proton source. To test these predictions, we investigated isotope effects (H2O versus D2O) on two stilbenoid-cleaving CCDs, Novosphingobium aromaticivorans oxygenase 2 (NOV2) and Neurospora crassa carotenoid oxygenase 1 (CAO1), using piceatannol as a substrate. NOV2 exhibited an inverse isotope effect (kH/kD ~ 0.6) in an air-saturated buffer, suggesting that solvent dissociates from iron during the catalytic cycle. By contrast, CAO1 displayed a normal isotope effect (kH/kD ~ 1.7), suggesting proton transfer in the rate-limiting step. X-ray absorption spectroscopy on NOV2 and CAO1 indicated that the protonation states of the iron ligands are unchanged within pH 6.5–8.5 and that the Fe(II)–aquo bond is minimally altered by substrate binding. In this study, we pinpointed themore »
- Authors:
-
- Case Western Reserve Univ., Cleveland, OH (United States)
- Brookhaven National Lab. (BNL), Upton, NY (United States); Case Western Reserve Univ., Cleveland, OH (United States)
- Publication Date:
- Research Org.:
- Brookhaven National Laboratory (BNL), Upton, NY (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); USDOE Office of Science (SC), Biological and Environmental Research (BER); National Institutes of Health (NIH)
- OSTI Identifier:
- 1679969
- Report Number(s):
- BNL-219982-2020-JAAM
Journal ID: ISSN 0021-9258
- Grant/Contract Number:
- SC0012704; GM103403; RR029205; AC02-06CH11357; AC02-76SF00515; P41-GM-103393; KP1605010; KC0401040; EB009998
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of Biological Chemistry
- Additional Journal Information:
- Journal Volume: 294; Journal Issue: 27; Journal ID: ISSN 0021-9258
- Publisher:
- American Society for Biochemistry and Molecular Biology
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 59 BASIC BIOLOGICAL SCIENCES; enzyme kinetics; iron; crystallography; carotenoid; dioxygenase; non-heme iron; O2 activation; solvent isotope effect; stilbenoid X-ray; absorption spectroscopy
Citation Formats
Khadka, Nimesh, Farquhar, Erik R., Hill, Hannah E., Shi, Wuxian, von Lintig, Johannes, and Kiser, Philip D. Evidence for distinct rate-limiting steps in the cleavage of alkenes by carotenoid cleavage dioxygenases. United States: N. p., 2019.
Web. doi:10.1074/jbc.ra119.007535.
Khadka, Nimesh, Farquhar, Erik R., Hill, Hannah E., Shi, Wuxian, von Lintig, Johannes, & Kiser, Philip D. Evidence for distinct rate-limiting steps in the cleavage of alkenes by carotenoid cleavage dioxygenases. United States. https://doi.org/10.1074/jbc.ra119.007535
Khadka, Nimesh, Farquhar, Erik R., Hill, Hannah E., Shi, Wuxian, von Lintig, Johannes, and Kiser, Philip D. Tue .
"Evidence for distinct rate-limiting steps in the cleavage of alkenes by carotenoid cleavage dioxygenases". United States. https://doi.org/10.1074/jbc.ra119.007535. https://www.osti.gov/servlets/purl/1679969.
@article{osti_1679969,
title = {Evidence for distinct rate-limiting steps in the cleavage of alkenes by carotenoid cleavage dioxygenases},
author = {Khadka, Nimesh and Farquhar, Erik R. and Hill, Hannah E. and Shi, Wuxian and von Lintig, Johannes and Kiser, Philip D.},
abstractNote = {Carotenoid cleavage dioxygenases (CCDs) use a nonheme Fe(II) cofactor to split alkene bonds of carotenoid and stilbenoid substrates. The iron centers of CCDs are typically five-coordinate in their resting states, with solvent occupying an exchangeable site. The involvement of this iron-bound solvent in CCD catalysis has not been experimentally addressed, but computational studies suggest two possible roles. 1) Solvent dissociation provides a coordination site for O2, or 2) solvent remains bound to iron but changes its equilibrium position to allow O2 binding and potentially acts as a proton source. To test these predictions, we investigated isotope effects (H2O versus D2O) on two stilbenoid-cleaving CCDs, Novosphingobium aromaticivorans oxygenase 2 (NOV2) and Neurospora crassa carotenoid oxygenase 1 (CAO1), using piceatannol as a substrate. NOV2 exhibited an inverse isotope effect (kH/kD ~ 0.6) in an air-saturated buffer, suggesting that solvent dissociates from iron during the catalytic cycle. By contrast, CAO1 displayed a normal isotope effect (kH/kD ~ 1.7), suggesting proton transfer in the rate-limiting step. X-ray absorption spectroscopy on NOV2 and CAO1 indicated that the protonation states of the iron ligands are unchanged within pH 6.5–8.5 and that the Fe(II)–aquo bond is minimally altered by substrate binding. In this study, we pinpointed the origin of the differential kinetic behaviors of NOV2 and CAO1 to a single amino acid difference near the solvent-binding site of iron, and X-ray crystallography revealed that the substitution alters binding of diffusible ligands to the iron center. We conclude that solvent-iron dissociation and proton transfer are both associated with the CCD catalytic mechanism.},
doi = {10.1074/jbc.ra119.007535},
journal = {Journal of Biological Chemistry},
number = 27,
volume = 294,
place = {United States},
year = {Tue May 28 00:00:00 EDT 2019},
month = {Tue May 28 00:00:00 EDT 2019}
}
Web of Science
Figures / Tables:
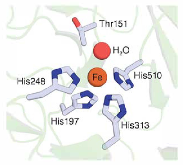 Figure 1: Resting state structure of the CA01 active site. The iron center of CAO1, a representative stilbene-cleaving CCD, is shown in the figure. Four histidine residues (His-197, -248, -313, and-510) coordinate the Fe(II) ion with an exchangeable aquo ligand as the fifth coordinating ligand. The figure was produced usingmore »
Figure 1: Resting state structure of the CA01 active site. The iron center of CAO1, a representative stilbene-cleaving CCD, is shown in the figure. Four histidine residues (His-197, -248, -313, and-510) coordinate the Fe(II) ion with an exchangeable aquo ligand as the fifth coordinating ligand. The figure was produced usingmore »
Works referenced in this record:
Structural basis of carotenoid cleavage: From bacteria to mammals
journal, November 2013
- Sui, Xuewu; Kiser, Philip D.; Lintig, Johannes von
- Archives of Biochemistry and Biophysics, Vol. 539, Issue 2
Utilization of Dioxygen by Carotenoid Cleavage Oxygenases
journal, October 2015
- Sui, Xuewu; Golczak, Marcin; Zhang, Jianye
- Journal of Biological Chemistry, Vol. 290, Issue 51
Structural and biological aspects of carotenoid cleavage
journal, August 2006
- Kloer, D. P.; Schulz, G. E.
- Cellular and Molecular Life Sciences, Vol. 63, Issue 19-20
Cloning, Expression, and Sequence Analysis of a Lignostilbene- α,β -dioxygenase Gene from Pseudomonas paucimobilis TMY1009
journal, January 1993
- Kamoda, Shigehiro; Saburi, Yoshimasa
- Bioscience, Biotechnology, and Biochemistry, Vol. 57, Issue 6
Carotenoid oxygenases: cleave it or leave it
journal, April 2003
- Giuliano, Giovanni; Al-Babili, Salim; von Lintig, Johannes
- Trends in Plant Science, Vol. 8, Issue 4
Carotene Oxygenases: A New Family of Double Bond Cleavage Enzymes
journal, January 2004
- Wyss, Adrian
- The Journal of Nutrition, Vol. 134, Issue 1
Retinal is formed from apo-carotenoids in Nostoc sp. PCC7120: in vitro characterization of an apo-carotenoid oxygenase
journal, August 2006
- Scherzinger, Daniel; Ruch, Sandra; Kloer, Daniel P.
- Biochemical Journal, Vol. 398, Issue 3
Specific Oxidative Cleavage of Carotenoids by VP14 of Maize
journal, June 1997
- Schwartz, S. H.
- Science, Vol. 276, Issue 5320
Related enzymes solve evolutionarily recurrent problems in the metabolism of carotenoids
journal, April 2005
- Moise, A.; Vonlintig, J.; Palczewski, K.
- Trends in Plant Science, Vol. 10, Issue 4
Strigolactones, a Novel Carotenoid-Derived Plant Hormone
journal, April 2015
- Al-Babili, Salim; Bouwmeester, Harro J.
- Annual Review of Plant Biology, Vol. 66, Issue 1
BCDO2 acts as a carotenoid scavenger and gatekeeper for the mitochondrial apoptotic pathway
journal, July 2012
- Lobo, G. P.; Isken, A.; Hoff, S.
- Development, Vol. 139, Issue 16
The Structure of a Retinal-Forming Carotenoid Oxygenase
journal, April 2005
- Kloer, D. P.
- Science, Vol. 308, Issue 5719
Structural Insights into Maize Viviparous14, a Key Enzyme in the Biosynthesis of the Phytohormone Abscisic Acid
journal, September 2010
- Messing, Simon A. J.; Gabelli, Sandra B.; Echeverria, Ignacia
- The Plant Cell, Vol. 22, Issue 9
Structure and Spectroscopy of Alkene-Cleaving Dioxygenases Containing an Atypically Coordinated Non-Heme Iron Center
journal, May 2017
- Sui, Xuewu; Weitz, Andrew C.; Farquhar, Erik R.
- Biochemistry, Vol. 56, Issue 22
Structure and mechanism of NOV1, a resveratrol-cleaving dioxygenase
journal, November 2016
- McAndrew, Ryan P.; Sathitsuksanoh, Noppadon; Mbughuni, Michael M.
- Proceedings of the National Academy of Sciences, Vol. 113, Issue 50
Structure and function of a lignostilbene-α,β-dioxygenase orthologue from Pseudomonas brassicacearum
journal, August 2018
- Loewen, Peter C.; Switala, Jacek; Wells, James P.
- BMC Biochemistry, Vol. 19, Issue 1
Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates
journal, February 2004
- Costas, Miquel; Mehn, Mark P.; Jensen, Michael P.
- Chemical Reviews, Vol. 104, Issue 2
Structure–function correlations in oxygen activating non-heme iron enzymes
journal, January 2005
- Neidig, Michael L.; Solomon, Edward I.
- Chemical Communications, Issue 47
Reaction Mechanism of Apocarotenoid Oxygenase (ACO): A DFT Study
journal, February 2008
- Borowski, Tomasz; Blomberg, Margareta R. A.; Siegbahn, Per E. M.
- Chemistry - A European Journal, Vol. 14, Issue 7
Hydrogen/deuterium fractionation factors of the aqueous ligand of cobalt in Co(H2O)62+ and Co(II)-substituted carbonic anhydrase
journal, March 1989
- Kassebaum, James W.; Silverman, David N.
- Journal of the American Chemical Society, Vol. 111, Issue 7
Inverse Solvent Isotope Effects Demonstrate Slow Aquo Release from Hypoxia Inducible Factor-Prolyl Hydroxylase (PHD2)
journal, August 2012
- Flagg, Shannon C.; Giri, Nitai; Pektas, Serap
- Biochemistry, Vol. 51, Issue 33
Insights into the pathogenesis of dominant retinitis pigmentosa associated with a D477G mutation in RPE65
journal, April 2018
- Choi, Elliot H.; Suh, Susie; Sander, Christopher L.
- Human Molecular Genetics, Vol. 27, Issue 13
Inverse Solvent Isotope Effects in the NAD-Malic Enzyme Reaction Are the Result of the Viscosity Difference between D2O and H2O: Implications for Solvent Isotope Effect Studies
journal, June 1995
- Karsten, William E.; Lai, Chung-Jeng; Cook, Paul F.
- Journal of the American Chemical Society, Vol. 117, Issue 22
Evidence from nitrogen-15 and solvent deuterium isotope effects on the chemical mechanism of adenosine deaminase
journal, November 1987
- Weiss, Paul M.; Cook, P. F.; Hermes, J. D.
- Biochemistry, Vol. 26, Issue 23
The Proton Inventory Techniqu
journal, January 1984
- Venkatasubban, K. S.; Schowen, Richard L.
- Critical Reviews in Biochemistry, Vol. 17, Issue 1
Mechanism of Nitrogenase H 2 Formation by Metal-Hydride Protonation Probed by Mediated Electrocatalysis and H/D Isotope Effects
journal, September 2017
- Khadka, Nimesh; Milton, Ross D.; Shaw, Sudipta
- Journal of the American Chemical Society, Vol. 139, Issue 38
MolProbity: More and better reference data for improved all-atom structure validation: PROTEIN SCIENCE.ORG
journal, November 2017
- Williams, Christopher J.; Headd, Jeffrey J.; Moriarty, Nigel W.
- Protein Science, Vol. 27, Issue 1
Large shifts in pKa values of lysine residues buried inside a protein
journal, March 2011
- Isom, D. G.; Castaneda, C. A.; Cannon, B. R.
- Proceedings of the National Academy of Sciences, Vol. 108, Issue 13
Nuclear Resonance Vibrational Spectroscopy Definition of O 2 Intermediates in an Extradiol Dioxygenase: Correlation to Crystallography and Reactivity
journal, November 2018
- Sutherlin, Kyle D.; Wasada-Tsutsui, Yuko; Mbughuni, Michael M.
- Journal of the American Chemical Society, Vol. 140, Issue 48
Preparation and characterization of metal-substituted carotenoid cleavage oxygenases
journal, June 2018
- Sui, Xuewu; Farquhar, Erik R.; Hill, Hannah E.
- JBIC Journal of Biological Inorganic Chemistry, Vol. 23, Issue 6
Analysis of Carotenoid Isomerase Activity in a Prototypical Carotenoid Cleavage Enzyme, Apocarotenoid Oxygenase (ACO)
journal, March 2014
- Sui, Xuewu; Kiser, Philip D.; Che, Tao
- Journal of Biological Chemistry, Vol. 289, Issue 18
XDS
journal, January 2010
- Kabsch, Wolfgang
- Acta Crystallographica Section D Biological Crystallography, Vol. 66, Issue 2
Phaser crystallographic software
journal, July 2007
- McCoy, Airlie J.; Grosse-Kunstleve, Ralf W.; Adams, Paul D.
- Journal of Applied Crystallography, Vol. 40, Issue 4
REFMAC 5 for the refinement of macromolecular crystal structures
journal, March 2011
- Murshudov, Garib N.; Skubák, Pavol; Lebedev, Andrey A.
- Acta Crystallographica Section D Biological Crystallography, Vol. 67, Issue 4
Features and development of Coot
journal, March 2010
- Emsley, P.; Lohkamp, B.; Scott, W. G.
- Acta Crystallographica Section D Biological Crystallography, Vol. 66, Issue 4
A New Generation of Crystallographic Validation Tools for the Protein Data Bank
journal, October 2011
- Read, Randy J.; Adams, Paul D.; Arendall, W. Bryan
- Structure, Vol. 19, Issue 10
Solvent isotope and viscosity effects on the steady-state kinetics of the flavoprotein nitroalkane oxidase
journal, May 2013
- Gadda, Giovanni; Fitzpatrick, Paul F.
- FEBS Letters, Vol. 587, Issue 17
Dioxygen Activation at Mononuclear Nonheme Iron Active Sites: Enzymes, Models, and Intermediates
journal, May 2004
- Costas, Miquel; Mehn, Mark P.; Jensen, Michael P.
- ChemInform, Vol. 35, Issue 21
PARP1 exhibits enhanced association and catalytic efficiency with γH2A.X-nucleosome
journal, December 2019
- Sharma, Deepti; De Falco, Louis; Padavattan, Sivaraman
- Nature Communications, Vol. 10, Issue 1
Structure—Function Correlations in Oxygen Activating Non-heme Iron Enzymes
journal, March 2006
- Neidig, Michael L.; Solomon, Edward I.
- ChemInform, Vol. 37, Issue 11
Works referencing / citing this record:
A modular pathway engineering strategy for the high-level production of β-ionone in Yarrowia lipolytica
journal, February 2020
- Lu, Yanping; Yang, Qingyu; Lin, Zhanglin
- Microbial Cell Factories, Vol. 19, Issue 1
A modular pathway engineering strategy for the high-level production of β-ionone in Yarrowia lipolytica
journal, February 2020
- Lu, Yanping; Yang, Qingyu; Lin, Zhanglin
- Microbial Cell Factories, Vol. 19, Issue 1
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal