High-Throughput Experimental Study of Wurtzite Mn 1– x Zn x O Alloys for Water Splitting Applications
Abstract
We used high-throughput experimental screening methods to unveil the physical and chemical properties of Mn1–xZnxO wurtzite alloys and identify their appropriate composition for effective water splitting application. The Mn1–xZnxO thin films were synthesized using combinatorial pulsed laser deposition, permitting for characterization of a wide range of compositions with x varying from 0 to 1. The solubility limit of ZnO in MnO was determined using the disappearing phase method from X-ray diffraction and X-ray fluorescence data and found to increase with decreasing substrate temperature due to kinetic limitations of the thin-film growth at relatively low temperature. Optical measurements indicate the strong reduction of the optical band gap down to 2.1 eV at x = 0.5 associated with the rock salt-to-wurtzite structural transition in Mn1–xZnxO alloys. Transmission electron microscopy results show evidence of a homogeneous wurtzite alloy system for a broad range of Mn1–xZnxO compositions above x = 0.4. The wurtzite Mn1–xZnxO samples with the 0.4 < x < 0.6 range were studied as anodes for photoelectrochemical water splitting, with a maximum current density of 340 μA cm–2 for 673 nm-thick films. These Mn1–xZnxO films were stable in pH = 10, showing no evidence of photocorrosion or degradation after 24 h undermore »
- Authors:
-
- Materials Science Center, National Renewable Energy Laboratory, Golden, Colorado 80401, United States
- Department of Materials Science and Engineering, The University of Arizona, Tucson, Arizona 85721, United States
- Department of Materials Science and Metallurgical Engineering, Indian Institute of Technology Hyderabad, Kandi, Sangareddy, Hyderabad 502285, India
- Materials Science Center, National Renewable Energy Laboratory, Golden, Colorado 80401, United States, Department of Metallurgical and Materials Engineering, Colorado School of Mines, Golden, Colorado 80401, United States
- Publication Date:
- Research Org.:
- Energy Frontier Research Centers (EFRC) (United States). Center for Next Generation of Materials by Design: Incorporating Metastability (CNGMD); National Renewable Energy Lab. (NREL), Golden, CO (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1508685
- Alternate Identifier(s):
- OSTI ID: 1510035; OSTI ID: 1512675
- Report Number(s):
- NREL/JA-5900-73457
Journal ID: ISSN 2470-1343
- Grant/Contract Number:
- AC36-08GO28308
- Resource Type:
- Published Article
- Journal Name:
- ACS Omega
- Additional Journal Information:
- Journal Name: ACS Omega Journal Volume: 4 Journal Issue: 4; Journal ID: ISSN 2470-1343
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; 36 MATERIALS SCIENCE; analytical chemistry; catalysts; combinatorial chemistry; crystal structure; electric properties; energy level; phase; phase transition; semiconductors; solid state electrochemistry; solubility; spectra; 71 CLASSICAL AND QUANTUM MECHANICS, GENERAL PHYSICS
Citation Formats
Ndione, Paul F., Ratcliff, Erin L., Dey, Suhash R., Warren, Emily L., Peng, Haowei, Holder, Aaron M., Lany, Stephan, Gorman, Brian P., Al-Jassim, Mowafak M., Deutsch, Todd G., Zakutayev, Andriy, and Ginley, David S. High-Throughput Experimental Study of Wurtzite Mn 1– x Zn x O Alloys for Water Splitting Applications. United States: N. p., 2019.
Web. doi:10.1021/acsomega.8b03347.
Ndione, Paul F., Ratcliff, Erin L., Dey, Suhash R., Warren, Emily L., Peng, Haowei, Holder, Aaron M., Lany, Stephan, Gorman, Brian P., Al-Jassim, Mowafak M., Deutsch, Todd G., Zakutayev, Andriy, & Ginley, David S. High-Throughput Experimental Study of Wurtzite Mn 1– x Zn x O Alloys for Water Splitting Applications. United States. https://doi.org/10.1021/acsomega.8b03347
Ndione, Paul F., Ratcliff, Erin L., Dey, Suhash R., Warren, Emily L., Peng, Haowei, Holder, Aaron M., Lany, Stephan, Gorman, Brian P., Al-Jassim, Mowafak M., Deutsch, Todd G., Zakutayev, Andriy, and Ginley, David S. Wed .
"High-Throughput Experimental Study of Wurtzite Mn 1– x Zn x O Alloys for Water Splitting Applications". United States. https://doi.org/10.1021/acsomega.8b03347.
@article{osti_1508685,
title = {High-Throughput Experimental Study of Wurtzite Mn 1– x Zn x O Alloys for Water Splitting Applications},
author = {Ndione, Paul F. and Ratcliff, Erin L. and Dey, Suhash R. and Warren, Emily L. and Peng, Haowei and Holder, Aaron M. and Lany, Stephan and Gorman, Brian P. and Al-Jassim, Mowafak M. and Deutsch, Todd G. and Zakutayev, Andriy and Ginley, David S.},
abstractNote = {We used high-throughput experimental screening methods to unveil the physical and chemical properties of Mn1–xZnxO wurtzite alloys and identify their appropriate composition for effective water splitting application. The Mn1–xZnxO thin films were synthesized using combinatorial pulsed laser deposition, permitting for characterization of a wide range of compositions with x varying from 0 to 1. The solubility limit of ZnO in MnO was determined using the disappearing phase method from X-ray diffraction and X-ray fluorescence data and found to increase with decreasing substrate temperature due to kinetic limitations of the thin-film growth at relatively low temperature. Optical measurements indicate the strong reduction of the optical band gap down to 2.1 eV at x = 0.5 associated with the rock salt-to-wurtzite structural transition in Mn1–xZnxO alloys. Transmission electron microscopy results show evidence of a homogeneous wurtzite alloy system for a broad range of Mn1–xZnxO compositions above x = 0.4. The wurtzite Mn1–xZnxO samples with the 0.4 < x < 0.6 range were studied as anodes for photoelectrochemical water splitting, with a maximum current density of 340 μA cm–2 for 673 nm-thick films. These Mn1–xZnxO films were stable in pH = 10, showing no evidence of photocorrosion or degradation after 24 h under water oxidation conditions. Doping Mn1–xZnxO materials with Ga dramatically increases the electrical conductivity of Mn1–xZnxO up to ~1.9 S/cm for x = 0.48, but these doped samples are not active in water splitting. Mott–Schottky and UPS/XPS measurements show that the presence of dopant atoms reduces the space charge region and increases the number of mid-gap surface states. Overall, this study demonstrates that Mn1–xZnxO alloys hold promise for photoelectrochemical water splitting, which could be enhanced with further tailoring of their electronic properties.},
doi = {10.1021/acsomega.8b03347},
journal = {ACS Omega},
number = 4,
volume = 4,
place = {United States},
year = {Wed Apr 24 00:00:00 EDT 2019},
month = {Wed Apr 24 00:00:00 EDT 2019}
}
https://doi.org/10.1021/acsomega.8b03347
Web of Science
Figures / Tables:
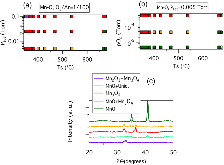 Figure 1: (a, b) Oxidation state maps obtained by XRD of the Mn−O system at (a) different total pressures as a function of substrate temperature and (b) different oxygen pressures as a function of substrate temperature. (c) Representative XRD patterns of the different phases, with the same color code asmore »
Figure 1: (a, b) Oxidation state maps obtained by XRD of the Mn−O system at (a) different total pressures as a function of substrate temperature and (b) different oxygen pressures as a function of substrate temperature. (c) Representative XRD patterns of the different phases, with the same color code asmore »
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal