Chiral DOTA chelators as an improved platform for biomedical imaging and therapy applications
Abstract
Despite established clinical utilisation, there is an increasing need for safer, more inert gadolinium-based contrast agents, and for chelators that react rapidly with radiometals. Here we report the syntheses of a series of chiral DOTA chelators and their corresponding metal complexes and reveal properties that transcend the parent DOTA compound. We incorporated symmetrical chiral substituents around the tetraaza ring, imparting enhanced rigidity to the DOTA cavity, enabling control over the range of stereoisomers of the lanthanide complexes. The Gd chiral DOTA complexes are shown to be orders of magnitude more inert to Gd release than [GdDOTA]¯. These compounds also exhibit very-fast water exchange rates in an optimal range for high field imaging. Radiolabeling studies with (Cu-64/Lu-177) also demonstrate faster labelling properties. These chiral DOTA chelators are alternative general platforms for the development of stable, high relaxivity contrast agents, and for radiometal complexes used for imaging and/or therapy.
- Authors:
-
- The Hong Kong Polytechnic Univ., Hong Kong (China)
- Massachusetts General Hospital and Harvard Medical School, Charlestown, MA (United States)
- Univ. of Pittsburgh, Pittsburgh, PA (United States)
- Publication Date:
- Research Org.:
- Univ. of Pittsburgh, PA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC)
- OSTI Identifier:
- 1511482
- Grant/Contract Number:
- SC0008833
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Nature Communications
- Additional Journal Information:
- Journal Volume: 9; Journal Issue: 1; Journal ID: ISSN 2041-1723
- Publisher:
- Nature Publishing Group
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 60 APPLIED LIFE SCIENCES
Citation Formats
Dai, Lixiong, Jones, Chloe M., Chan, Wesley Ting Kwok, Pham, Tiffany A., Ling, Xiaoxi, Gale, Eric M., Rotile, Nicholas J., Tai, William Chi-Shing, Anderson, Carolyn J., Caravan, Peter, and Law, Ga -Lai. Chiral DOTA chelators as an improved platform for biomedical imaging and therapy applications. United States: N. p., 2018.
Web. doi:10.1038/s41467-018-03315-8.
Dai, Lixiong, Jones, Chloe M., Chan, Wesley Ting Kwok, Pham, Tiffany A., Ling, Xiaoxi, Gale, Eric M., Rotile, Nicholas J., Tai, William Chi-Shing, Anderson, Carolyn J., Caravan, Peter, & Law, Ga -Lai. Chiral DOTA chelators as an improved platform for biomedical imaging and therapy applications. United States. https://doi.org/10.1038/s41467-018-03315-8
Dai, Lixiong, Jones, Chloe M., Chan, Wesley Ting Kwok, Pham, Tiffany A., Ling, Xiaoxi, Gale, Eric M., Rotile, Nicholas J., Tai, William Chi-Shing, Anderson, Carolyn J., Caravan, Peter, and Law, Ga -Lai. Tue .
"Chiral DOTA chelators as an improved platform for biomedical imaging and therapy applications". United States. https://doi.org/10.1038/s41467-018-03315-8. https://www.osti.gov/servlets/purl/1511482.
@article{osti_1511482,
title = {Chiral DOTA chelators as an improved platform for biomedical imaging and therapy applications},
author = {Dai, Lixiong and Jones, Chloe M. and Chan, Wesley Ting Kwok and Pham, Tiffany A. and Ling, Xiaoxi and Gale, Eric M. and Rotile, Nicholas J. and Tai, William Chi-Shing and Anderson, Carolyn J. and Caravan, Peter and Law, Ga -Lai},
abstractNote = {Despite established clinical utilisation, there is an increasing need for safer, more inert gadolinium-based contrast agents, and for chelators that react rapidly with radiometals. Here we report the syntheses of a series of chiral DOTA chelators and their corresponding metal complexes and reveal properties that transcend the parent DOTA compound. We incorporated symmetrical chiral substituents around the tetraaza ring, imparting enhanced rigidity to the DOTA cavity, enabling control over the range of stereoisomers of the lanthanide complexes. The Gd chiral DOTA complexes are shown to be orders of magnitude more inert to Gd release than [GdDOTA]¯. These compounds also exhibit very-fast water exchange rates in an optimal range for high field imaging. Radiolabeling studies with (Cu-64/Lu-177) also demonstrate faster labelling properties. These chiral DOTA chelators are alternative general platforms for the development of stable, high relaxivity contrast agents, and for radiometal complexes used for imaging and/or therapy.},
doi = {10.1038/s41467-018-03315-8},
journal = {Nature Communications},
number = 1,
volume = 9,
place = {United States},
year = {Tue Feb 27 00:00:00 EST 2018},
month = {Tue Feb 27 00:00:00 EST 2018}
}
Web of Science
Figures / Tables:
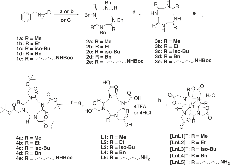 Fig. 1 : Synthetic procedures of chiral DOTA complexes. The chiral cyclens were synthesised from chiral aziridines followed by the incorporation of protected acetate arms and subsequent deprotections to give the final ligands. (a) TsOH/EtOH, RT (1a); (b) BF3·Et2O/Benzene, reflux (1b – 1d), 24 h; (c) TsOH/ACN, RT (1b, 1e);more »
Fig. 1 : Synthetic procedures of chiral DOTA complexes. The chiral cyclens were synthesised from chiral aziridines followed by the incorporation of protected acetate arms and subsequent deprotections to give the final ligands. (a) TsOH/EtOH, RT (1a); (b) BF3·Et2O/Benzene, reflux (1b – 1d), 24 h; (c) TsOH/ACN, RT (1b, 1e);more »
Works referenced in this record:
MR Contrast Agents for Liver Imaging: What, When, How
journal, November 2006
- Gandhi, Sunil N.; Brown, Michèle A.; Wong, James G.
- RadioGraphics, Vol. 26, Issue 6
Monovalent and Bivalent Fibrin-specific MRI Contrast Agents for Detection of Thrombus
journal, June 2008
- Nair, Shrikumar A.; Kolodziej, Andrew F.; Bhole, Gandhali
- Angewandte Chemie International Edition, Vol. 47, Issue 26
Multilocus Binding Increases the Relaxivity of Protein-Bound MRI Contrast Agents
journal, October 2005
- Zhang, Zhaoda; Greenfield, Matthew T.; Spiller, Marga
- Angewandte Chemie International Edition, Vol. 44, Issue 41
The Interaction of MS-325 with Human Serum Albumin and Its Effect on Proton Relaxation Rates
journal, March 2002
- Caravan, Peter; Cloutier, Normand J.; Greenfield, Matthew T.
- Journal of the American Chemical Society, Vol. 124, Issue 12
High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadolinium-based Contrast Material
journal, March 2014
- Kanda, Tomonori; Ishii, Kazunari; Kawaguchi, Hiroki
- Radiology, Vol. 270, Issue 3
T1-Weighted Hypersignal in the Deep Cerebellar Nuclei After Repeated Administrations of Gadolinium-Based Contrast Agents in Healthy Rats: Difference Between Linear and Macrocyclic Agents
journal, January 2015
- Robert, Philippe; Lehericy, Stéphane; Grand, Sylvie
- Investigative Radiology, Vol. 50, Issue 8
Nephrogenic Systemic Fibrosis: Suspected Causative Role of Gadodiamide Used for Contrast-Enhanced Magnetic Resonance Imaging
journal, August 2006
- Marckmann, Peter; Skov, Lone; Rossen, Kristian
- Journal of the American Society of Nephrology, Vol. 17, Issue 9
Biodistribution of Radiolabeled, Formulated Gadopentetate, Gadoteridol, Gadoterate, and Gadodiamide in Mice and Rats
journal, January 1995
- Tweedle, Michael F.; Wedeking, Paul; Kumar, Krishan
- Investigative Radiology, Vol. 30, Issue 6
Nephrogenic Systemic Fibrosis and Class Labeling of Gadolinium-based Contrast Agents by the Food and Drug Administration
journal, October 2012
- Yang, Lucie; Krefting, Ira; Gorovets, Alex
- Radiology, Vol. 265, Issue 1
Analysis of the Conformational Behavior and Stability of the SAP and TSAP Isomers of Lanthanide(III) NB-DOTA-Type Chelates
journal, September 2011
- Tircso, Gyula; Webber, Benjamin C.; Kucera, Benjamin E.
- Inorganic Chemistry, Vol. 50, Issue 17
Biodistribution of gadolinium-based contrast agents, including gadolinium deposition
journal, December 2009
- Aime, Silvio; Caravan, Peter
- Journal of Magnetic Resonance Imaging, Vol. 30, Issue 6
In-Cell Protein Structures from 2D NMR Experiments
journal, June 2016
- Müntener, Thomas; Häussinger, Daniel; Selenko, Philipp
- The Journal of Physical Chemistry Letters, Vol. 7, Issue 14
Direct Measurement of the Mn(II) Hydration State in Metal Complexes and Metalloproteins through 17 O NMR Line Widths
journal, October 2013
- Gale, Eric M.; Zhu, Jiang; Caravan, Peter
- Journal of the American Chemical Society, Vol. 135, Issue 49
Chiral Tetraazamacrocycles Having Four Pendant-Arms
journal, June 2009
- Kamioka, Seiji; Takahashi, Takashi; Kawauchi, Susumu
- Organic Letters, Vol. 11, Issue 11
Isomerism in Benzyl-DOTA Derived Bifunctional Chelators: Implications for Molecular Imaging
journal, January 2015
- Payne, Katherine M.; Woods, Mark
- Bioconjugate Chemistry, Vol. 26, Issue 2
High Relaxivity Magnetic Resonance Imaging Contrast Agents Part 1: Impact of Single Donor Atom Substitution on Relaxivity of Serum Albumin-Bound Gadolinium Complexes
journal, January 2010
- Dumas, Stéphane; Jacques, Vincent; Sun, Wei-Chuan
- Investigative Radiology, Vol. 45, Issue 10
Hepatobiliary-specific MR Contrast Agents: Role in Imaging the Liver and Biliary Tree
journal, October 2009
- Seale, Melanie K.; Catalano, Onofrio A.; Saini, Sanjay
- RadioGraphics, Vol. 29, Issue 6
Gd(III) complexes of poly(hydroxymethyl)substituted derivatives of 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
journal, May 2001
- Anelli, Pier Lucio; Beltrami, Andrea; Franzini, Maurizio
- Inorganica Chimica Acta, Vol. 317, Issue 1-2
Increasing the Chemical-Shift Dispersion of Unstructured Proteins with a Covalent Lanthanide Shift Reagent
journal, October 2016
- Göbl, Christoph; Resch, Moritz; Strickland, Madeleine
- Angewandte Chemie International Edition, Vol. 55, Issue 47
Coordinating Radiometals of Copper, Gallium, Indium, Yttrium, and Zirconium for PET and SPECT Imaging of Disease
journal, May 2010
- Wadas, Thaddeus J.; Wong, Edward H.; Weisman, Gary R.
- Chemical Reviews, Vol. 110, Issue 5
Gadolinium – a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?
journal, January 2006
- Grobner, Thomas
- Nephrology Dialysis Transplantation, Vol. 21, Issue 4
Optimization of the Relaxivity of MRI Contrast Agents: Effect of Poly(ethylene glycol) Chains on the Water-Exchange Rates of Gd III Complexes
journal, October 2001
- Doble, Dan M. J.; Botta, Mauro; Wang, Jay
- Journal of the American Chemical Society, Vol. 123, Issue 43
Synergistic Effect of Human Serum Albumin and Fullerene on Gd-DO3A for Tumor-Targeting Imaging
journal, April 2016
- Zhang, Ying; Zou, Toujun; Guan, Mirong
- ACS Applied Materials & Interfaces, Vol. 8, Issue 18
Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms
journal, April 2016
- Rogosnitzky, Moshe; Branch, Stacy
- BioMetals, Vol. 29, Issue 3
Towards the Rational Design of Magnetic Resonance Imaging Contrast Agents: Isolation of the Two Coordination Isomers of Lanthanide DOTA-Type Complexes
journal, December 2003
- Woods, Mark; Kovacs, Zoltan; Zhang, Shanrong
- Angewandte Chemie International Edition, Vol. 42, Issue 47
Residual or Retained Gadolinium: Practical Implications for Radiologists and Our Patients
journal, June 2015
- Kanal, Emanuel; Tweedle, Michael F.
- Radiology, Vol. 275, Issue 3
Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review
journal, March 2008
- Port, Marc; Idée, Jean-Marc; Medina, Christelle
- BioMetals, Vol. 21, Issue 4
Polymethylated DOTA Ligands. 1. Synthesis of Rigidified Ligands and Studies on the Effects of Alkyl Substitution on Acid−Base Properties and Conformational Mobility
journal, December 2002
- Ranganathan, Ramachandran S.; Pillai, Radhakrishna K.; Raju, Natarajan
- Inorganic Chemistry, Vol. 41, Issue 25
Albumin Binding, Relaxivity, and Water Exchange Kinetics of the Diastereoisomers of MS-325, a Gadolinium(III)-Based Magnetic Resonance Angiography Contrast Agent
journal, August 2007
- Caravan, Peter; Parigi, Giacomo; Chasse, Jaclyn M.
- Inorganic Chemistry, Vol. 46, Issue 16
Strategies for increasing the sensitivity of gadolinium based MRI contrast agents
journal, January 2006
- Caravan, Peter
- Chemical Society Reviews, Vol. 35, Issue 6
Properties, Solution State Behavior, and Crystal Structures of Chelates of DOTMA
journal, September 2011
- Aime, Silvio; Botta, Mauro; Garda, Zoltán
- Inorganic Chemistry, Vol. 50, Issue 17
Determination of free gadolinium(3+) as a cyclohexanediaminetetraacetic acid complex by reversed-phase HPLC in ionic gadolinium(III) chelates
journal, January 1994
- Kumar, Krishan.; Sukumaran, K. V.; Tweedle, M. F.
- Analytical Chemistry, Vol. 66, Issue 2
Chiral Recognition of α-Amino Acids by an Optically Active (2 S ,5 S ,8 S ,11 S )-2,5,8,11-Tetraethyl Cyclen Cobalt(III) Complex
journal, January 2011
- Tashiro, Shohei; Ogura, Yasuyo; Tsuboyama, Sei
- Inorganic Chemistry, Vol. 50, Issue 1
Strategies for Optimizing Water-Exchange Rates of Lanthanide-Based Contrast Agents for Magnetic Resonance Imaging
journal, August 2013
- Siriwardena-Mahanama, Buddhima; Allen, Matthew
- Molecules, Vol. 18, Issue 8
Gadolinium deposition in the brain: summary of evidence and recommendations
journal, July 2017
- Gulani, Vikas; Calamante, Fernando; Shellock, Frank G.
- The Lancet Neurology, Vol. 16, Issue 7
Influence of molecular parameters and increasing magnetic field strength on relaxivity of gadolinium- and manganese-based T 1 contrast agents
journal, March 2009
- Caravan, Peter; Farrar, Christian T.; Frullano, Luca
- Contrast Media & Molecular Imaging, Vol. 4, Issue 2
Linear Gadolinium-Based Contrast Agents Are Associated With Brain Gadolinium Retention in Healthy Rats
journal, January 2016
- Robert, Philippe; Violas, Xavier; Grand, Sylvie
- Investigative Radiology, Vol. 51, Issue 2
Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats: Differences in Gd Retention Between cGBCAs
journal, July 2017
- Bussi, Simona; Coppo, Alessandra; Botteron, Catherine
- Journal of Magnetic Resonance Imaging, Vol. 47, Issue 3
NMR, Relaxometric, and Structural Studies of the Hydration and Exchange Dynamics of Cationic Lanthanide Complexes of Macrocyclic Tetraamide Ligands
journal, June 1999
- Aime, Silvio; Barge, Alessandro; Bruce, James I.
- Journal of the American Chemical Society, Vol. 121, Issue 24
Comparative study of the physicochemical properties of six clinical low molecular weight gadolinium contrast agents
journal, January 2006
- Laurent, Sophie; Elst, Luce Vander; Muller, Robert N.
- Contrast Media & Molecular Imaging, Vol. 1, Issue 3
Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications
journal, September 1999
- Caravan, Peter; Ellison, Jeffrey J.; McMurry, Thomas J.
- Chemical Reviews, Vol. 99, Issue 9
Solution Dynamics and Stability of Lanthanide(III) ( S ) - 2-( p -Nitrobenzyl)DOTA Complexes
journal, May 2004
- Woods, Mark; Kovacs, Zoltan; Kiraly, Robert
- Inorganic Chemistry, Vol. 43, Issue 9
Lanthanide(III) Complexes with a Reinforced Cyclam Ligand Show Unprecedented Kinetic Inertness
journal, December 2014
- Rodríguez-Rodríguez, Aurora; Esteban-Gómez, David; Tripier, Raphaël
- Journal of the American Chemical Society, Vol. 136, Issue 52
Matching chelators to radiometals for radiopharmaceuticals
journal, January 2014
- Price, Eric W.; Orvig, Chris
- Chem. Soc. Rev., Vol. 43, Issue 1
Solution and Solid-State Characterization of Highly Rigid, Eight-Coordinate Lanthanide(III) Complexes of a Macrocyclic Tetrabenzylphosphinate
journal, October 1994
- Aime, Silvio; Batsanov, Andrei S.; Botta, Mauro
- Inorganic Chemistry, Vol. 33, Issue 21
Aggregation in Amphiphilic Macrocycle-Substituted Gd 3+ DOTA-Type Chelates Is Affected by the Regiochemistry of Substitution
journal, February 2015
- Webber, Benjamin C.; Cassino, Claudio; Botta, Mauro
- Inorganic Chemistry, Vol. 54, Issue 5
The importance of water exchange rates in the design of responsive agents for MRI
journal, April 2013
- Sherry, A. Dean; Wu, Yunkou
- Current Opinion in Chemical Biology, Vol. 17, Issue 2
Heteroditopic Binding of Magnetic Resonance Contrast Agents for Increased Relaxivity
journal, February 2011
- Zhang, Zhaoda; Kolodziej, Andrew F.; Greenfield, Matthew T.
- Angewandte Chemie International Edition, Vol. 50, Issue 11
Coupling Fast Water Exchange to Slow Molecular Tumbling in Gd 3+ Chelates: Why Faster Is Not Always Better
journal, July 2013
- Avedano, Stefano; Botta, Mauro; Haigh, Julian S.
- Inorganic Chemistry, Vol. 52, Issue 15
Structural and Dynamic Parameters Obtained from 17 O NMR, EPR, and NMRD Studies of Monomeric and Dimeric Gd 3+ Complexes of Interest in Magnetic Resonance Imaging: An Integrated and Theoretically Self-Consistent Approach 1
journal, January 1996
- Powell, D. Hugh; Dhubhghaill, Orla M. Ni; Pubanz, Dirk
- Journal of the American Chemical Society, Vol. 118, Issue 39
Gadolinium-based Contrast Agent Accumulates in the Brain Even in Subjects without Severe Renal Dysfunction: Evaluation of Autopsy Brain Specimens with Inductively Coupled Plasma Mass Spectroscopy
journal, July 2015
- Kanda, Tomonori; Fukusato, Toshio; Matsuda, Megumi
- Radiology, Vol. 276, Issue 1
Gd(DOTAlaP): Exploring the Boundaries of Fast Water Exchange in Gadolinium-Based Magnetic Resonance Imaging Contrast Agents
journal, June 2014
- Boros, Eszter; Karimi, Shima; Kenton, Nathaniel
- Inorganic Chemistry, Vol. 53, Issue 13
Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging
journal, June 2015
- McDonald, Robert J.; McDonald, Jennifer S.; Kallmes, David F.
- Radiology, Vol. 275, Issue 3
Circularly Polarized Luminescence of Curium: A New Characterization of the 5f Actinide Complexes
journal, September 2012
- Law, Ga-Lai; Andolina, Christopher M.; Xu, Jide
- Journal of the American Chemical Society, Vol. 134, Issue 37
Polymethylated DOTA Ligands. 2. Synthesis of Rigidified Lanthanide Chelates and Studies on the Effect of Alkyl Substitution on Conformational Mobility and Relaxivity
journal, December 2002
- Ranganathan, Ramachandran S.; Raju, Natarajan; Fan, Helen
- Inorganic Chemistry, Vol. 41, Issue 25
New Class of Bright and Highly Stable Chiral Cyclen Europium Complexes for Circularly Polarized Luminescence Applications
journal, August 2016
- Dai, Lixiong; Lo, Wai-Sum; Coates, Ian D.
- Inorganic Chemistry, Vol. 55, Issue 17
Monovalent and Bivalent Fibrin-specific MRI Contrast Agents for Detection of Thrombus
journal, June 2008
- Nair, Shrikumar A.; Kolodziej, Andrew F.; Bhole, Gandhali
- Angewandte Chemie, Vol. 120, Issue 26
Towards the Rational Design of Magnetic Resonance Imaging Contrast Agents: Isolation of the Two Coordination Isomers of Lanthanide DOTA-Type Complexes
journal, December 2003
- Woods, Mark; Kovacs, Zoltan; Zhang, Shanrong
- Angewandte Chemie, Vol. 115, Issue 47
Multilocus Binding Increases the Relaxivity of Protein-Bound MRI Contrast Agents
journal, October 2005
- Zhang, Zhaoda; Greenfield, Matthew T.; Spiller, Marga
- Angewandte Chemie, Vol. 117, Issue 41
Strategies for Increasing the Sensitivity of Gadolinium Based MRI Contrast Agents
journal, August 2006
- Caravan, Peter
- ChemInform, Vol. 37, Issue 35
Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?
journal, January 2007
- Elster, A. D.
- Yearbook of Diagnostic Radiology, Vol. 2007
In-Cell Protein Structures from 2D NMR Experiments
text, January 2016
- Müntener, Thomas; Häussinger, Daniel; Selenko, Philipp
- American Chemical Society
Heteroditopic Binding of Magnetic Resonance Contrast Agents for Increased Relaxivity
journal, February 2011
- Zhang, Zhaoda; Kolodziej, Andrew F.; Greenfield, Matthew T.
- Angewandte Chemie, Vol. 123, Issue 11
Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?
journal, June 2006
- Grobner, Thomas
- Nephrology Dialysis Transplantation, Vol. 21, Issue 6
Multilocus Binding Increases the Relaxivity of Protein-Bound MRI Contrast Agents
journal, October 2005
- Zhang, Zhaoda; Greenfield, Matthew T.; Spiller, Marga
- Angewandte Chemie, Vol. 117, Issue 41
Monovalent and Bivalent Fibrin-specific MRI Contrast Agents for Detection of Thrombus
journal, June 2008
- Nair, Shrikumar A.; Kolodziej, Andrew F.; Bhole, Gandhali
- Angewandte Chemie, Vol. 120, Issue 26
Heteroditopic Binding of Magnetic Resonance Contrast Agents for Increased Relaxivity
journal, February 2011
- Zhang, Zhaoda; Kolodziej, Andrew F.; Greenfield, Matthew T.
- Angewandte Chemie, Vol. 123, Issue 11
Towards the Rational Design of Magnetic Resonance Imaging Contrast Agents: Isolation of the Two Coordination Isomers of Lanthanide DOTA-Type Complexes
journal, December 2003
- Woods, Mark; Kovacs, Zoltan; Zhang, Shanrong
- Angewandte Chemie International Edition, Vol. 42, Issue 47
Influence of molecular parameters and increasing magnetic field strength on relaxivity of gadolinium- and manganese-based T 1 contrast agents
journal, March 2009
- Caravan, Peter; Farrar, Christian T.; Frullano, Luca
- Contrast Media & Molecular Imaging, Vol. 4, Issue 2
Efficiency, thermodynamic and kinetic stability of marketed gadolinium chelates and their possible clinical consequences: a critical review
journal, March 2008
- Port, Marc; Idée, Jean-Marc; Medina, Christelle
- BioMetals, Vol. 21, Issue 4
Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms
journal, April 2016
- Rogosnitzky, Moshe; Branch, Stacy
- BioMetals, Vol. 29, Issue 3
The importance of water exchange rates in the design of responsive agents for MRI
journal, April 2013
- Sherry, A. Dean; Wu, Yunkou
- Current Opinion in Chemical Biology, Vol. 17, Issue 2
Brain gadolinium deposition, hyperintense MRI signals, and resonance contrast agents
journal, October 2018
- Costa, Antonella; Ronchi, Anna; Pigatto, Paolo D.
- Magnetic Resonance Imaging, Vol. 52
Cyclic tetramers of optically active aziridines: 1,4,7,10-tetrabenzyl-2,5,8,11-tetra-(R)-ethyl-1,4,7,10-tetraazacyclododecane
journal, January 1970
- Tsuboyama, Sei; Tsuboyama, Kaoru; Higashi, Iwami
- Tetrahedron Letters, Vol. 11, Issue 16
Determination of free gadolinium(3+) as a cyclohexanediaminetetraacetic acid complex by reversed-phase HPLC in ionic gadolinium(III) chelates
journal, January 1994
- Kumar, Krishan.; Sukumaran, K. V.; Tweedle, M. F.
- Analytical Chemistry, Vol. 66, Issue 2
New Class of Bright and Highly Stable Chiral Cyclen Europium Complexes for Circularly Polarized Luminescence Applications
journal, August 2016
- Dai, Lixiong; Lo, Wai-Sum; Coates, Ian D.
- Inorganic Chemistry, Vol. 55, Issue 17
In-Cell Protein Structures from 2D NMR Experiments
journal, June 2016
- Müntener, Thomas; Häussinger, Daniel; Selenko, Philipp
- The Journal of Physical Chemistry Letters, Vol. 7, Issue 14
Isomerism in Benzyl-DOTA Derived Bifunctional Chelators: Implications for Molecular Imaging
journal, January 2015
- Payne, Katherine M.; Woods, Mark
- Bioconjugate Chemistry, Vol. 26, Issue 2
Gadolinium(III) Chelates as MRI Contrast Agents: Structure, Dynamics, and Applications
journal, September 1999
- Caravan, Peter; Ellison, Jeffrey J.; McMurry, Thomas J.
- Chemical Reviews, Vol. 99, Issue 9
Solution and Solid-State Characterization of Highly Rigid, Eight-Coordinate Lanthanide(III) Complexes of a Macrocyclic Tetrabenzylphosphinate
journal, October 1994
- Aime, Silvio; Batsanov, Andrei S.; Botta, Mauro
- Inorganic Chemistry, Vol. 33, Issue 21
Polymethylated DOTA Ligands. 2. Synthesis of Rigidified Lanthanide Chelates and Studies on the Effect of Alkyl Substitution on Conformational Mobility and Relaxivity
journal, December 2002
- Ranganathan, Ramachandran S.; Raju, Natarajan; Fan, Helen
- Inorganic Chemistry, Vol. 41, Issue 25
Solution Dynamics and Stability of Lanthanide(III) ( S ) - 2-( p -Nitrobenzyl)DOTA Complexes
journal, May 2004
- Woods, Mark; Kovacs, Zoltan; Kiraly, Robert
- Inorganic Chemistry, Vol. 43, Issue 9
Properties, Solution State Behavior, and Crystal Structures of Chelates of DOTMA
journal, September 2011
- Aime, Silvio; Botta, Mauro; Garda, Zoltán
- Inorganic Chemistry, Vol. 50, Issue 17
Analysis of the Conformational Behavior and Stability of the SAP and TSAP Isomers of Lanthanide(III) NB-DOTA-Type Chelates
journal, September 2011
- Tircso, Gyula; Webber, Benjamin C.; Kucera, Benjamin E.
- Inorganic Chemistry, Vol. 50, Issue 17
Gd(DOTAlaP): Exploring the Boundaries of Fast Water Exchange in Gadolinium-Based Magnetic Resonance Imaging Contrast Agents
journal, June 2014
- Boros, Eszter; Karimi, Shima; Kenton, Nathaniel
- Inorganic Chemistry, Vol. 53, Issue 13
Aggregation in Amphiphilic Macrocycle-Substituted Gd 3+ DOTA-Type Chelates Is Affected by the Regiochemistry of Substitution
journal, February 2015
- Webber, Benjamin C.; Cassino, Claudio; Botta, Mauro
- Inorganic Chemistry, Vol. 54, Issue 5
Albumin Binding, Relaxivity, and Water Exchange Kinetics of the Diastereoisomers of MS-325, a Gadolinium(III)-Based Magnetic Resonance Angiography Contrast Agent
journal, August 2007
- Caravan, Peter; Parigi, Giacomo; Chasse, Jaclyn M.
- Inorganic Chemistry, Vol. 46, Issue 16
Optimization of the Relaxivity of MRI Contrast Agents: Effect of Poly(ethylene glycol) Chains on the Water-Exchange Rates of Gd III Complexes
journal, October 2001
- Doble, Dan M. J.; Botta, Mauro; Wang, Jay
- Journal of the American Chemical Society, Vol. 123, Issue 43
The Interaction of MS-325 with Human Serum Albumin and Its Effect on Proton Relaxation Rates
journal, March 2002
- Caravan, Peter; Cloutier, Normand J.; Greenfield, Matthew T.
- Journal of the American Chemical Society, Vol. 124, Issue 12
Circularly Polarized Luminescence of Curium: A New Characterization of the 5f Actinide Complexes
journal, September 2012
- Law, Ga-Lai; Andolina, Christopher M.; Xu, Jide
- Journal of the American Chemical Society, Vol. 134, Issue 37
Direct Measurement of the Mn(II) Hydration State in Metal Complexes and Metalloproteins through 17 O NMR Line Widths
journal, October 2013
- Gale, Eric M.; Zhu, Jiang; Caravan, Peter
- Journal of the American Chemical Society, Vol. 135, Issue 49
Lanthanide(III) Complexes with a Reinforced Cyclam Ligand Show Unprecedented Kinetic Inertness
journal, December 2014
- Rodríguez-Rodríguez, Aurora; Esteban-Gómez, David; Tripier, Raphaël
- Journal of the American Chemical Society, Vol. 136, Issue 52
Structural and Dynamic Parameters Obtained from 17 O NMR, EPR, and NMRD Studies of Monomeric and Dimeric Gd 3+ Complexes of Interest in Magnetic Resonance Imaging: An Integrated and Theoretically Self-Consistent Approach 1
journal, January 1996
- Powell, D. Hugh; Dhubhghaill, Orla M. Ni; Pubanz, Dirk
- Journal of the American Chemical Society, Vol. 118, Issue 39
Chiral Tetraazamacrocycles Having Four Pendant-Arms
journal, June 2009
- Kamioka, Seiji; Takahashi, Takashi; Kawauchi, Susumu
- Organic Letters, Vol. 11, Issue 11
Lanthanide( III ) chelates for NMR biomedical applications
journal, January 1998
- Aime, Silvio; Botta, Mauro; Fasano, Mauro
- Chem. Soc. Rev., Vol. 27, Issue 1
Strategies for increasing the sensitivity of gadolinium based MRI contrast agents
journal, January 2006
- Caravan, Peter
- Chemical Society Reviews, Vol. 35, Issue 6
Analysis of the isomer ratios of polymethylated-DOTA complexes and the implications on protein structural studies
journal, January 2016
- Opina, Ana Christina L.; Strickland, Madeleine; Lee, Yong-Sok
- Dalton Transactions, Vol. 45, Issue 11
Gadolinium—a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis?
journal, June 2006
- Grobner, Thomas
- Nephrology Dialysis Transplantation, Vol. 21, Issue 6
Commentary on T1-Weighted Hypersignal in the Deep Cerebellar Nuclei After Repeated Administrations of Gadolinium-Based Contrast Agents in Healthy Rats
journal, August 2015
- Runge, Val M.
- Investigative Radiology, Vol. 50, Issue 8
Nephrogenic Systemic Fibrosis and Class Labeling of Gadolinium-based Contrast Agents by the Food and Drug Administration
journal, October 2012
- Yang, Lucie; Krefting, Ira; Gorovets, Alex
- Radiology, Vol. 265, Issue 1
Residual or Retained Gadolinium: Practical Implications for Radiologists and Our Patients
journal, June 2015
- Kanal, Emanuel; Tweedle, Michael F.
- Radiology, Vol. 275, Issue 3
MR Contrast Agents for Liver Imaging: What, When, How
journal, November 2006
- Gandhi, Sunil N.; Brown, Michèle A.; Wong, James G.
- RadioGraphics, Vol. 26, Issue 6
Hepatobiliary-specific MR Contrast Agents: Role in Imaging the Liver and Biliary Tree
journal, October 2009
- Seale, Melanie K.; Catalano, Onofrio A.; Saini, Sanjay
- RadioGraphics, Vol. 29, Issue 6
Outcomes of Abbreviated MRI (Ab-MRI) for Women of any Breast Cancer Risk and Breast Density in a Community Academic Setting
journal, July 2022
- Kennard, Kaitlyn; Wang, Olivia; Kjelstrom, Stephanie
- Annals of Surgical Oncology, Vol. 29, Issue 10
Strategies for Optimizing Water-Exchange Rates of Lanthanide-Based Contrast Agents for Magnetic Resonance Imaging
journal, August 2013
- Siriwardena-Mahanama, Buddhima; Allen, Matthew
- Molecules, Vol. 18, Issue 8
Works referencing / citing this record:
A Sterically Overcrowded, Isopropyl‐Substituted, Lanthanide‐Chelating Tag for Protein Pseudocontact Shift NMR Spectroscopy: Synthesis of its Macrocyclic Scaffold and Benchmarking on Ubiquitin S57 C and hCA II S166 C
journal, August 2019
- Joss, Daniel; Bertrams, Maria‐Sophie; Häussinger, Daniel
- Chemistry – A European Journal, Vol. 25, Issue 51
Effect of Ligand Chirality and Hyperconjugation on the Thermodynamic Stability of a Tris(aquated) Gd III Complex: Synthesis, Characterization, and T 1 -Weighted Phantom MR Image Study: Effect of Ligand Chirality and Hyperconjugation on the Thermodynamic Stability of a Tris(aquated) GdIII Complex: Synthesis, Characterization, and T1-W
journal, May 2019
- Khannam, Mahmuda; Sahoo, Suban K.; Mukherjee, Chandan
- European Journal of Inorganic Chemistry, Vol. 2019, Issue 20
The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations: The Use of the Macrocyclic Chelator DOTA in Radiochemical Separations
journal, November 2019
- Baranyai, Zsolt; Tircsó, Gyula; Rösch, Frank
- European Journal of Inorganic Chemistry, Vol. 2020, Issue 1
A Bishydrated, Eight–Coordinate Gd(III) Complex with Very Fast Water Exchange: Synthesis, Characterization, and Phantom MR Imaging
journal, July 2018
- Phukan, Bedika; Malikidogo, Kyangwi P.; Bonnet, Célia S.
- ChemistrySelect, Vol. 3, Issue 27
Ligand design strategies to increase stability of gadolinium-based magnetic resonance imaging contrast agents
journal, March 2019
- Clough, Thomas J.; Jiang, Lijun; Wong, Ka-Leung
- Nature Communications, Vol. 10, Issue 1
Synthesis of chiral nine and twelve-membered cyclic polyamines from natural building blocks
journal, January 2019
- Müntener, Thomas; Thommen, Fabienne; Joss, Daniel
- Chemical Communications, Vol. 55, Issue 32
The use of the macrocyclic chelator DOTA in radiochemical separations
text, January 2020
- Baranyai, Zsolt; Tircsó, Gyula; Rösch, Frank
- Wiley-VCH
Synthesis of chiral nine and twelve-membered cyclic polyamines from natural building blocks
text, January 2019
- Thomas, Müntener,; Fabienne, Thommen,; Daniel, Joss,
- Royal Society of Chemistry
A Sterically Overcrowded, Isopropyl-Substituted, Lanthanide-Chelating Tag for Protein Pseudocontact Shift NMR Spectroscopy: Synthesis of its Macrocyclic Scaffold and Benchmarking on Ubiquitin S57 C and hCA II S166 C
text, January 2019
- Joss, Daniel; Bertrams, Maria-Sophie; Häussinger, Daniel
- Wiley
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal