A Biochemical Nickel(I) State Supports Nucleophilic Alkyl Addition: A Roadmap for Methyl Reactivity in Acetyl Coenzyme A Synthase
Abstract
Nickel-containing enzymes such as methyl coenzyme M reductase (MCR) and carbon monoxide dehydrogenase/acetyl coenzyme A synthase (CODH/ACS) play a critical role in global energy conversion reactions, with significant contributions to carbon-centered processes. These enzymes are implied to cycle through a series of nickel-based organometallic intermediates during catalysis, though identification of these intermediates remains challenging. In this work, we have developed and characterized a nickel-containing metalloprotein that models the methyl-bound organometallic intermediates proposed in the native enzymes. Using a nickel(I)-substituted azurin mutant, we demonstrate that alkyl binding occurs via nucleophilic addition of methyl iodide as a methyl donor. The paramagnetic NiIII-CH3 species initially generated can be rapidly reduced to a high-spin NiII-CH3 species in the presence of exogenous reducing agent, following a reaction sequence analogous to that proposed for ACS. These two distinct bioorganometallic species have been characterized by optical, EPR, XAS, and MCD spectroscopy, and the overall mechanism describing methyl reactivity with nickel azurin has been quantitatively modeled using global kinetic simulations. A comparison between the nickel azurin protein system and existing ACS model compounds is presented. NiIII-CH3 Az is only the second example of two-electron addition of methyl iodide to a NiI center to give an isolable species andmore »
- Authors:
-
- Department of Chemistry and Biochemistry, The Ohio State University, 100 W. 18th Avenue, Columbus, Ohio 43210, United States
- Department of Chemistry, University of Michigan, 930 N. University Avenue, Ann Arbor, Michigan 48109, United States
- Department of Chemistry, Trinity University, One Trinity Place, San Antonio, Texas 78212, United States
- Publication Date:
- Research Org.:
- The Ohio State Univ., Columbus, OH (United States); Trinity Univ., San Antonio, TX (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES); ACS Petroleum Research Fund (ACS PRF) (United States); National Science Foundation (NSF); National Inst. of Health (NIH) (United States)
- OSTI Identifier:
- 1495569
- Alternate Identifier(s):
- OSTI ID: 1508801; OSTI ID: 1542555
- Grant/Contract Number:
- SC0018020; 57403-DNI6; CHE-1565766; GM120641
- Resource Type:
- Published Article
- Journal Name:
- Inorganic Chemistry
- Additional Journal Information:
- Journal Name: Inorganic Chemistry Journal Volume: 58 Journal Issue: 14; Journal ID: ISSN 0020-1669
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Manesis, Anastasia C., Musselman, Bradley W., Keegan, Brenna C., Shearer, Jason, Lehnert, Nicolai, and Shafaat, Hannah S. A Biochemical Nickel(I) State Supports Nucleophilic Alkyl Addition: A Roadmap for Methyl Reactivity in Acetyl Coenzyme A Synthase. United States: N. p., 2019.
Web. doi:10.1021/acs.inorgchem.8b03546.
Manesis, Anastasia C., Musselman, Bradley W., Keegan, Brenna C., Shearer, Jason, Lehnert, Nicolai, & Shafaat, Hannah S. A Biochemical Nickel(I) State Supports Nucleophilic Alkyl Addition: A Roadmap for Methyl Reactivity in Acetyl Coenzyme A Synthase. United States. https://doi.org/10.1021/acs.inorgchem.8b03546
Manesis, Anastasia C., Musselman, Bradley W., Keegan, Brenna C., Shearer, Jason, Lehnert, Nicolai, and Shafaat, Hannah S. Thu .
"A Biochemical Nickel(I) State Supports Nucleophilic Alkyl Addition: A Roadmap for Methyl Reactivity in Acetyl Coenzyme A Synthase". United States. https://doi.org/10.1021/acs.inorgchem.8b03546.
@article{osti_1495569,
title = {A Biochemical Nickel(I) State Supports Nucleophilic Alkyl Addition: A Roadmap for Methyl Reactivity in Acetyl Coenzyme A Synthase},
author = {Manesis, Anastasia C. and Musselman, Bradley W. and Keegan, Brenna C. and Shearer, Jason and Lehnert, Nicolai and Shafaat, Hannah S.},
abstractNote = {Nickel-containing enzymes such as methyl coenzyme M reductase (MCR) and carbon monoxide dehydrogenase/acetyl coenzyme A synthase (CODH/ACS) play a critical role in global energy conversion reactions, with significant contributions to carbon-centered processes. These enzymes are implied to cycle through a series of nickel-based organometallic intermediates during catalysis, though identification of these intermediates remains challenging. In this work, we have developed and characterized a nickel-containing metalloprotein that models the methyl-bound organometallic intermediates proposed in the native enzymes. Using a nickel(I)-substituted azurin mutant, we demonstrate that alkyl binding occurs via nucleophilic addition of methyl iodide as a methyl donor. The paramagnetic NiIII-CH3 species initially generated can be rapidly reduced to a high-spin NiII-CH3 species in the presence of exogenous reducing agent, following a reaction sequence analogous to that proposed for ACS. These two distinct bioorganometallic species have been characterized by optical, EPR, XAS, and MCD spectroscopy, and the overall mechanism describing methyl reactivity with nickel azurin has been quantitatively modeled using global kinetic simulations. A comparison between the nickel azurin protein system and existing ACS model compounds is presented. NiIII-CH3 Az is only the second example of two-electron addition of methyl iodide to a NiI center to give an isolable species and the first to be formed in a biologically relevant system. These results highlight the divergent reactivity of nickel across the two intermediates, with implications for likely reaction mechanisms and catalytically relevant states in the native ACS enzyme.},
doi = {10.1021/acs.inorgchem.8b03546},
journal = {Inorganic Chemistry},
number = 14,
volume = 58,
place = {United States},
year = {Thu Feb 21 00:00:00 EST 2019},
month = {Thu Feb 21 00:00:00 EST 2019}
}
https://doi.org/10.1021/acs.inorgchem.8b03546
Web of Science
Figures / Tables:
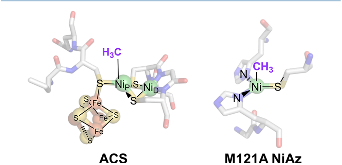 Figure 1: Proposed nickel methyl intermediates in ACS and NiAz.
Figure 1: Proposed nickel methyl intermediates in ACS and NiAz.
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal