Selective Charging Behavior in an Ionic Mixture Electrolyte-Supercapacitor System for Higher Energy and Power
- Authors:
-
- Norwegian Univ. of Science and Technology, Trondheim (Norway); Drexel Univ., Philadelphia, PA (United States)
- Norwegian Univ. of Science and Technology, Trondheim (Norway)
- SINTEF Materials and Chemistry, Oslo (Norway)
- Drexel Univ., Philadelphia, PA (United States)
- Univ. of California, Riverside, CA (United States)
- SINTEF Materials and Chemistry, Trondheim (Norway)
- Publication Date:
- Research Org.:
- Lawrence Berkeley National Lab. (LBNL), Berkeley, CA (United States). National Energy Research Scientific Computing Center (NERSC); Energy Frontier Research Centers (EFRC) (United States). Fluid Interface Reactions, Structures and Transport Center (FIRST); Oak Ridge National Lab. (ORNL), Oak Ridge, TN (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1489029
- Alternate Identifier(s):
- OSTI ID: 1470145; OSTI ID: 1494890
- Grant/Contract Number:
- ERKCC61; AC05-00OR22725
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of the American Chemical Society
- Additional Journal Information:
- Journal Volume: 139; Journal Issue: 51; Journal ID: ISSN 0002-7863
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; catalysis (heterogeneous), solar (fuels), energy storage (including batteries and capacitors), hydrogen and fuel cells, electrodes - solar, mechanical behavior, charge transport, materials and chemistry by design, synthesis (novel materials)
Citation Formats
Wang, Xuehang, Mehandzhiyski, Aleksandar Yordanov, Arstad, Bjørnar, Van Aken, Katherine L., Mathis, Tyler S., Gallegos, Alejandro, Tian, Ziqi, Ren, Dingding, Sheridan, Edel, Grimes, Brian Arthur, Jiang, De-en, Wu, Jianzhong, Gogotsi, Yury, and Chen, De. Selective Charging Behavior in an Ionic Mixture Electrolyte-Supercapacitor System for Higher Energy and Power. United States: N. p., 2017.
Web. doi:10.1021/jacs.7b10693.
Wang, Xuehang, Mehandzhiyski, Aleksandar Yordanov, Arstad, Bjørnar, Van Aken, Katherine L., Mathis, Tyler S., Gallegos, Alejandro, Tian, Ziqi, Ren, Dingding, Sheridan, Edel, Grimes, Brian Arthur, Jiang, De-en, Wu, Jianzhong, Gogotsi, Yury, & Chen, De. Selective Charging Behavior in an Ionic Mixture Electrolyte-Supercapacitor System for Higher Energy and Power. United States. https://doi.org/10.1021/jacs.7b10693
Wang, Xuehang, Mehandzhiyski, Aleksandar Yordanov, Arstad, Bjørnar, Van Aken, Katherine L., Mathis, Tyler S., Gallegos, Alejandro, Tian, Ziqi, Ren, Dingding, Sheridan, Edel, Grimes, Brian Arthur, Jiang, De-en, Wu, Jianzhong, Gogotsi, Yury, and Chen, De. Wed .
"Selective Charging Behavior in an Ionic Mixture Electrolyte-Supercapacitor System for Higher Energy and Power". United States. https://doi.org/10.1021/jacs.7b10693. https://www.osti.gov/servlets/purl/1489029.
@article{osti_1489029,
title = {Selective Charging Behavior in an Ionic Mixture Electrolyte-Supercapacitor System for Higher Energy and Power},
author = {Wang, Xuehang and Mehandzhiyski, Aleksandar Yordanov and Arstad, Bjørnar and Van Aken, Katherine L. and Mathis, Tyler S. and Gallegos, Alejandro and Tian, Ziqi and Ren, Dingding and Sheridan, Edel and Grimes, Brian Arthur and Jiang, De-en and Wu, Jianzhong and Gogotsi, Yury and Chen, De},
abstractNote = {},
doi = {10.1021/jacs.7b10693},
journal = {Journal of the American Chemical Society},
number = 51,
volume = 139,
place = {United States},
year = {Wed Nov 29 00:00:00 EST 2017},
month = {Wed Nov 29 00:00:00 EST 2017}
}
Free Publicly Available Full Text
Publisher's Version of Record
Other availability
Cited by: 76 works
Citation information provided by
Web of Science
Web of Science
Figures / Tables:
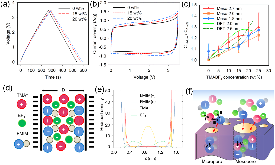 Figure 1: (a,b) GCD at 1 A/g and CV curves at 10 mV/s of the mesopore-rich carbon Meso-2.0 nm. (c) Relation between normalized capacitance and TMABF4 concentration with three mesopore-rich carbon materials. (d) Schematic representation of a room-temperature ionic mixture in a charged slit pore: TMA+ and BF4− ions aremore »
Figure 1: (a,b) GCD at 1 A/g and CV curves at 10 mV/s of the mesopore-rich carbon Meso-2.0 nm. (c) Relation between normalized capacitance and TMABF4 concentration with three mesopore-rich carbon materials. (d) Schematic representation of a room-temperature ionic mixture in a charged slit pore: TMA+ and BF4− ions aremore »
All figures and tables
(3 total)
Save to My Library
You must Sign In or Create an Account in order to save documents to your library.
Works referenced in this record:
Materials for electrochemical capacitors
journal, November 2008
- Simon, Patrice; Gogotsi, Yury
- Nature Materials, Vol. 7, Issue 11
True Performance Metrics in Electrochemical Energy Storage
journal, November 2011
- Gogotsi, Y.; Simon, P.
- Science, Vol. 334, Issue 6058
Efficient storage mechanisms for building better supercapacitors
journal, May 2016
- Salanne, M.; Rotenberg, B.; Naoi, K.
- Nature Energy, Vol. 1, Issue 6
Formulation of Ionic-Liquid Electrolyte To Expand the Voltage Window of Supercapacitors
journal, March 2015
- Van Aken, Katherine L.; Beidaghi, Majid; Gogotsi, Yury
- Angewandte Chemie International Edition, Vol. 54, Issue 16
Ionic-liquid materials for the electrochemical challenges of the future
journal, July 2009
- Armand, Michel; Endres, Frank; MacFarlane, Douglas R.
- Nature Materials, Vol. 8, Issue 8, p. 621-629
Anomalous Increase in Carbon Capacitance at Pore Sizes Less Than 1 Nanometer
journal, September 2006
- Chmiola, J.
- Science, Vol. 313, Issue 5794
Relation between the Ion Size and Pore Size for an Electric Double-Layer Capacitor
journal, March 2008
- Largeot, Celine; Portet, Cristelle; Chmiola, John
- Journal of the American Chemical Society, Vol. 130, Issue 9
Geometrically confined favourable ion packing for high gravimetric capacitance in carbon–ionic liquid supercapacitors
journal, January 2016
- Wang, Xuehang; Zhou, Haitao; Sheridan, Edel
- Energy & Environmental Science, Vol. 9, Issue 1
Carbons and Electrolytes for Advanced Supercapacitors
journal, February 2014
- Béguin, François; Presser, Volker; Balducci, Andrea
- Advanced Materials, Vol. 26, Issue 14, p. 2219-2251
Solvent effect on the ion adsorption from ionic liquid electrolyte into sub-nanometer carbon pores
journal, November 2009
- Lin, R.; Huang, P.; Ségalini, J.
- Electrochimica Acta, Vol. 54, Issue 27
Ion Dynamics in Porous Carbon Electrodes in Supercapacitors Using in Situ Infrared Spectroelectrochemistry
journal, August 2013
- Richey, Francis W.; Dyatkin, Boris; Gogotsi, Yury
- Journal of the American Chemical Society, Vol. 135, Issue 34
On the Dynamics of Charging in Nanoporous Carbon-Based Supercapacitors
journal, January 2014
- Péan, Clarisse; Merlet, Céline; Rotenberg, Benjamin
- ACS Nano, Vol. 8, Issue 2
Direct observation of ion dynamics in supercapacitor electrodes using in situ diffusion NMR spectroscopy
journal, February 2017
- Forse, Alexander C.; Griffin, John M.; Merlet, Céline
- Nature Energy, Vol. 2, Issue 3
Confinement, Desolvation, And Electrosorption Effects on the Diffusion of Ions in Nanoporous Carbon Electrodes
journal, September 2015
- Pean, Clarisse; Daffos, Barbara; Rotenberg, Benjamin
- Journal of the American Chemical Society, Vol. 137, Issue 39
Boosted Supercapacitive Energy with High Rate Capability of aCarbon Framework with Hierarchical Pore Structure in an Ionic Liquid
journal, October 2016
- Wang, Xuehang; Zhou, Haitao; Lou, Fengliu
- ChemSusChem, Vol. 9, Issue 21
Double-Layer in Ionic Liquids: Paradigm Change?
journal, May 2007
- Kornyshev, Alexei A.
- The Journal of Physical Chemistry B, Vol. 111, Issue 20
Steric effects in adsorption of ions from mixed electrolytes into microporous carbon
journal, February 2012
- Segalini, Julie; Iwama, Etsuro; Taberna, Pierre-Louis
- Electrochemistry Communications, Vol. 15, Issue 1
On the molecular origin of supercapacitance in nanoporous carbon electrodes
journal, March 2012
- Merlet, Céline; Rotenberg, Benjamin; Madden, Paul A.
- Nature Materials, Vol. 11, Issue 4
Electrochemical Quartz Crystal Microbalance (EQCM) Study of Ion Dynamics in Nanoporous Carbons
journal, June 2014
- Tsai, Wan-Yu; Taberna, Pierre-Louis; Simon, Patrice
- Journal of the American Chemical Society, Vol. 136, Issue 24
NMR Study of Ion Dynamics and Charge Storage in Ionic Liquid Supercapacitors
journal, May 2015
- Forse, Alexander C.; Griffin, John M.; Merlet, Céline
- Journal of the American Chemical Society, Vol. 137, Issue 22
Highly confined ions store charge more efficiently in supercapacitors
journal, October 2013
- Merlet, C.; Péan, C.; Rotenberg, B.
- Nature Communications, Vol. 4, Issue 1
Desolvation of Ions in Subnanometer Pores and Its Effect on Capacitance and Double-Layer Theory
journal, April 2008
- Chmiola, John; Largeot, Celine; Taberna, Pierre-Louis
- Angewandte Chemie, Vol. 120, Issue 18
Importance of Ion Packing on the Dynamics of Ionic Liquids during Micropore Charging
journal, December 2015
- He, Yadong; Qiao, Rui; Vatamanu, Jenel
- The Journal of Physical Chemistry Letters, Vol. 7, Issue 1
Can ionophobic nanopores enhance the energy storage capacity of electric-double-layer capacitors containing nonaqueous electrolytes?
journal, August 2016
- Lian, Cheng; Liu, Honglai; Henderson, Douglas
- Journal of Physics: Condensed Matter, Vol. 28, Issue 41
New Perspectives on the Charging Mechanisms of Supercapacitors
journal, April 2016
- Forse, Alexander C.; Merlet, Céline; Griffin, John M.
- Journal of the American Chemical Society, Vol. 138, Issue 18
Rapid and Accurate Estimation of Densities of Room-Temperature Ionic Liquids and Salts
journal, March 2007
- Ye, Chengfeng; Shreeve, Jean'ne M.
- The Journal of Physical Chemistry A, Vol. 111, Issue 8
Room-Temperature Ionic Liquids. Solvents for Synthesis and Catalysis
journal, August 1999
- Welton, Thomas
- Chemical Reviews, Vol. 99, Issue 8, p. 2071-2084
Solvent-free ionic liquids as in situ probes for assessing the effect of ion size on the performance of electrical double layer capacitors
journal, November 2006
- Ania, C. O.; Pernak, J.; Stefaniak, F.
- Carbon, Vol. 44, Issue 14
Capacitive Energy Storage from −50 to 100 °C Using an Ionic Liquid Electrolyte
journal, September 2011
- Lin, Rongying; Taberna, Pierre-Louis; Fantini, Sébastien
- The Journal of Physical Chemistry Letters, Vol. 2, Issue 19
Outstanding performance of activated graphene based supercapacitors in ionic liquid electrolyte from −50 to 80°C
journal, May 2013
- Tsai, Wan-Yu; Lin, Rongying; Murali, Shanthi
- Nano Energy, Vol. 2, Issue 3
Cation−π Interactions in Simple Aromatics: Electrostatics Provide a Predictive Tool
journal, January 1996
- Mecozzi, Sandro; West, Anthony P.; Dougherty, Dennis A.
- Journal of the American Chemical Society, Vol. 118, Issue 9
Competitive pi interactions and hydrogen bonding within imidazolium ionic liquids
journal, January 2014
- Matthews, Richard P.; Welton, Tom; Hunt, Patricia A.
- Physical Chemistry Chemical Physics, Vol. 16, Issue 7
A Computational Study of Cation−π Interactions vs Salt Bridges in Aqueous Media: Implications for Protein Engineering
journal, January 2000
- Gallivan, Justin P.; Dougherty, Dennis A.
- Journal of the American Chemical Society, Vol. 122, Issue 5
Exploring electrolyte organization in supercapacitor electrodes with solid-state NMR
journal, February 2013
- Deschamps, Michaël; Gilbert, Edouard; Azais, Philippe
- Nature Materials, Vol. 12, Issue 4
In situ NMR and electrochemical quartz crystal microbalance techniques reveal the structure of the electrical double layer in supercapacitors
journal, June 2015
- Griffin, John M.; Forse, Alexander C.; Tsai, Wan-Yu
- Nature Materials, Vol. 14, Issue 8
Real-Time NMR Studies of Electrochemical Double-Layer Capacitors
journal, December 2011
- Wang, Hao; Köster, Thomas K. -J.; Trease, Nicole M.
- Journal of the American Chemical Society, Vol. 133, Issue 48
Predictive thermodynamics for condensed phases
journal, January 2005
- Glasser, Leslie; Jenkins, H. Donald Brooke
- Chemical Society Reviews, Vol. 34, Issue 10
Why Are Ionic Liquids Liquid? A Simple Explanation Based on Lattice and Solvation Energies
journal, October 2006
- Krossing, Ingo; Slattery, John M.; Daguenet, Corinne
- Journal of the American Chemical Society, Vol. 128, Issue 41
Works referencing / citing this record:
A universal strategy to obtain highly redox-active porous carbons for efficient energy storage
journal, January 2020
- Song, Ziyang; Miao, Ling; Li, Liangchun
- Journal of Materials Chemistry A, Vol. 8, Issue 7
Density functional calculations of efficient H 2 separation from impurity gases (H 2 , N 2 , H 2 O, CO, Cl 2 , and CH 4 ) via bilayer g-C 3 N 4 membrane
journal, April 2019
- Guo, Yuan; Tang, Chunmei; Wang, Xinbo
- Chinese Physics B, Vol. 28, Issue 4
Side-chain effects on the capacitive behaviour of ionic liquids in microporous electrodes
journal, August 2019
- Gallegos, Alejandro; Lian, Cheng; Dyatkin, Boris
- Molecular Physics, Vol. 117, Issue 23-24
Ionic liquid electrolytes in electric double layer capacitors
journal, July 2019
- Yin, Li; Li, Shu; Liu, Xiaohong
- Science China Materials, Vol. 62, Issue 11
Co 2+ induced phase transformation from δ- to α-MnO 2 and their hierarchical α-MnO 2 @δ-MnO 2 nanostructures for efficient asymmetric supercapacitors
journal, January 2019
- Guo, Yu; Li, Lei; Song, Li
- Journal of Materials Chemistry A, Vol. 7, Issue 20
Highly accessible hierarchical porous carbon from a bi-functional ionic liquid bulky gel: high-performance electrochemical double layer capacitors
journal, January 2019
- Yan, Yang; Hao, Xiao-Feng; Gao, Li-guo
- Journal of Materials Chemistry A, Vol. 7, Issue 44
Rational Design of Carbon‐Rich Materials for Energy Storage and Conversion
journal, October 2018
- Kong, Debin; Gao, Yang; Xiao, Zhichang
- Advanced Materials, Vol. 31, Issue 45
A first-principles roadmap and limits to design efficient supercapacitor electrode materials
journal, January 2019
- Ali, Basant A.; Allam, Nageh K.
- Physical Chemistry Chemical Physics, Vol. 21, Issue 32
Using inorganic dynamic porogens for preparing high-surface-area capacitive carbons with tailored micropores
journal, January 2019
- Dong, Xiao-Ling; Li, Wen-Cui; Jiang, Biao
- Journal of Materials Chemistry A, Vol. 7, Issue 2
Side-chain effects on the capacitive behaviour of ionic liquids in microporous electrodes
text, January 2019
- Gallegos, Alejandro; Lian, Cheng; Dyatkin, Boris
- Taylor & Francis
Figures / Tables found in this record:
Figures/Tables have been extracted from DOE-funded journal article accepted manuscripts.

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal