Interaction of antidiabetic α-glucosidase inhibitors and gut bacteria α-glucosidase
Abstract
Abstract Carbohydrate hydrolyzing α‐glucosidases are commonly found in microorganisms present in the human intestine microbiome. We have previously reported crystal structures of an α‐glucosidase from the human gut bacterium Blaubia ( Ruminococcus ) obeum ( Ro ‐αG1) and its substrate preference/specificity switch. This novel member of the GH31 family is a structural homolog of human intestinal maltase‐glucoamylase (MGAM) and sucrase–isomaltase (SI) with a highly conserved active site that is predicted to be common in Ro ‐αG1 homologs among other species that colonize the human gut. In this report, we present structures of Ro ‐αG1 in complex with the antidiabetic α‐glucosidase inhibitors voglibose, miglitol, and acarbose and supporting binding data. The in vitro binding of these antidiabetic drugs to Ro ‐αG1 suggests the potential for unintended in vivo crossreaction of the α‐glucosidase inhibitors to bacterial α‐glucosidases that are present in gut microorganism communities. Moreover, analysis of these drug‐bound enzyme structures could benefit further antidiabetic drug development.
- Authors:
-
- Argonne National Lab. (ANL), Argonne, IL (United States)
- Argonne National Lab. (ANL), Argonne, IL (United States); Univ. of Chicago, Chicago, IL (United States)
- Publication Date:
- Research Org.:
- Argonne National Laboratory (ANL), Argonne, IL (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Biological and Environmental Research (BER)
- OSTI Identifier:
- 1491841
- Alternate Identifier(s):
- OSTI ID: 1459683
- Grant/Contract Number:
- AC02-06CH11357
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Protein Science
- Additional Journal Information:
- Journal Volume: 27; Journal Issue: 8; Journal ID: ISSN 0961-8368
- Publisher:
- The Protein Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 59 BASIC BIOLOGICAL SCIENCES; acarbose; anti-diabetic drug; human gut microbiome; miglitol; substrate/inhibitor selection; voglibose; α-glucosidase; α-glucosidase inhibitor
Citation Formats
Tan, Kemin, Tesar, Christine, Wilton, Rosemarie, Jedrzejczak, Robert P., and Joachimiak, Andrzej. Interaction of antidiabetic α-glucosidase inhibitors and gut bacteria α-glucosidase. United States: N. p., 2018.
Web. doi:10.1002/pro.3444.
Tan, Kemin, Tesar, Christine, Wilton, Rosemarie, Jedrzejczak, Robert P., & Joachimiak, Andrzej. Interaction of antidiabetic α-glucosidase inhibitors and gut bacteria α-glucosidase. United States. https://doi.org/10.1002/pro.3444
Tan, Kemin, Tesar, Christine, Wilton, Rosemarie, Jedrzejczak, Robert P., and Joachimiak, Andrzej. Tue .
"Interaction of antidiabetic α-glucosidase inhibitors and gut bacteria α-glucosidase". United States. https://doi.org/10.1002/pro.3444. https://www.osti.gov/servlets/purl/1491841.
@article{osti_1491841,
title = {Interaction of antidiabetic α-glucosidase inhibitors and gut bacteria α-glucosidase},
author = {Tan, Kemin and Tesar, Christine and Wilton, Rosemarie and Jedrzejczak, Robert P. and Joachimiak, Andrzej},
abstractNote = {Abstract Carbohydrate hydrolyzing α‐glucosidases are commonly found in microorganisms present in the human intestine microbiome. We have previously reported crystal structures of an α‐glucosidase from the human gut bacterium Blaubia ( Ruminococcus ) obeum ( Ro ‐αG1) and its substrate preference/specificity switch. This novel member of the GH31 family is a structural homolog of human intestinal maltase‐glucoamylase (MGAM) and sucrase–isomaltase (SI) with a highly conserved active site that is predicted to be common in Ro ‐αG1 homologs among other species that colonize the human gut. In this report, we present structures of Ro ‐αG1 in complex with the antidiabetic α‐glucosidase inhibitors voglibose, miglitol, and acarbose and supporting binding data. The in vitro binding of these antidiabetic drugs to Ro ‐αG1 suggests the potential for unintended in vivo crossreaction of the α‐glucosidase inhibitors to bacterial α‐glucosidases that are present in gut microorganism communities. Moreover, analysis of these drug‐bound enzyme structures could benefit further antidiabetic drug development.},
doi = {10.1002/pro.3444},
journal = {Protein Science},
number = 8,
volume = 27,
place = {United States},
year = {Tue May 15 00:00:00 EDT 2018},
month = {Tue May 15 00:00:00 EDT 2018}
}
Web of Science
Figures / Tables:
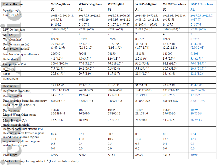 Table 1: Crystallographic statistics
Table 1: Crystallographic statistics
Works referenced in this record:
A Genomic View of the Human-Bacteroides thetaiotaomicron Symbiosis
journal, March 2003
- Xu, J.
- Science, Vol. 299, Issue 5615
The Polymerase Incomplete Primer Extension (PIPE) Method Applied to High-Throughput Cloning and Site-Directed Mutagenesis
book, January 2009
- Klock, Heath E.; Lesley, Scott A.
- Methods in Molecular Biology
Structure of the Sulfolobus solfataricus α-Glucosidase: Implications for Domain Conservation and Substrate Recognition in GH31
journal, May 2006
- Ernst, Heidi A.; Lo Leggio, Leila; Willemoës, Martin
- Journal of Molecular Biology, Vol. 358, Issue 4
In vitro inhibition of glycogen-degrading enzymes and glycosidases by six-membered sugar mimics and their evaluation in cell cultures
journal, August 2008
- Kuriyama, Chinami; Kamiyama, Ogusa; Ikeda, Kyoko
- Bioorganic & Medicinal Chemistry, Vol. 16, Issue 15
Cell-wall carbohydrates and their modification as a resource for biofuels
journal, May 2008
- Pauly, Markus; Keegstra, Kenneth
- The Plant Journal, Vol. 54, Issue 4, p. 559-568
Reclassification of Ruminococcus obeum as Blautia obeum comb. nov.
journal, December 2014
- Lawson, P. A.; Finegold, S. M.
- INTERNATIONAL JOURNAL OF SYSTEMATIC AND EVOLUTIONARY MICROBIOLOGY, Vol. 65, Issue Pt 3
Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota
journal, December 2015
- Forslund, Kristoffer; Hildebrand, Falk; Nielsen, Trine
- Nature, Vol. 528, Issue 7581
The synthesis and biological evaluation of 1-C-alkyl-l-arabinoiminofuranoses, a novel class of α-glucosidase inhibitors
journal, January 2011
- Natori, Yoshihiro; Imahori, Tatsushi; Murakami, Keiichi
- Bioorganic & Medicinal Chemistry Letters, Vol. 21, Issue 2
The gastrointestinal microbiota as a site for the biotransformation of drugs
journal, November 2008
- Sousa, Tiago; Paterson, Ronnie; Moore, Vanessa
- International Journal of Pharmaceutics, Vol. 363, Issue 1-2
Comparative Metagenomics Revealed Commonly Enriched Gene Sets in Human Gut Microbiomes
journal, January 2007
- Kurokawa, Ken; Itoh, Takehiko; Kuwahara, Tomomi
- DNA Research, Vol. 14, Issue 4
Alpha-glucosidase inhibitors 2012 – cardiovascular considerations and trial evaluation
journal, April 2012
- Standl, Eberhard; Schnell, Oliver
- Diabetes and Vascular Disease Research, Vol. 9, Issue 3
Gut Pharmacomicrobiomics: the tip of an iceberg of complex interactions between drugs and gut-associated microbes
journal, January 2012
- Saad, Rama; Rizkallah, Mariam R.; Aziz, Ramy K.
- Gut Pathogens, Vol. 4, Issue 1
The Structural Biology Center 19ID undulator beamline: facility specifications and protein crystallographic results
journal, December 2005
- Rosenbaum, Gerd; Alkire, Randy W.; Evans, Gwyndaf
- Journal of Synchrotron Radiation, Vol. 13, Issue 1
Human gut microbiota plays a role in the metabolism of drugs
journal, September 2016
- Jourova, Lenka; Anzenbacher, Pavel; Anzenbacherova, Eva
- Biomedical Papers, Vol. 160, Issue 3
Novel α-glucosidase from human gut microbiome: substrate specificities and their switch
journal, October 2010
- Tan, Kemin; Tesar, Christine; Wilton, Rosemarie
- The FASEB Journal, Vol. 24, Issue 10
Subsite Mapping of the Human Pancreatic α-Amylase Active Site through Structural, Kinetic, and Mutagenesis Techniques † , ‡
journal, April 2000
- Brayer, Gary D.; Sidhu, Gary; Maurus, Robert
- Biochemistry, Vol. 39, Issue 16
The effect of gut microbiota on drug metabolism
journal, May 2013
- Kang, Mi Jeong; Kim, Hyung Gyun; Kim, Jin Sung
- Expert Opinion on Drug Metabolism & Toxicology, Vol. 9, Issue 10
The Human Microbiome Project
journal, October 2007
- Turnbaugh, Peter J.; Ley, Ruth E.; Hamady, Micah
- Nature, Vol. 449, Issue 7164
Xenobiotics Shape the Physiology and Gene Expression of the Active Human Gut Microbiome
journal, January 2013
- Maurice, Corinne Ferrier; Haiser, Henry Joseph; Turnbaugh, Peter James
- Cell, Vol. 152, Issue 1-2
New Glucosidase Inhibitors from an Ayurvedic Herbal Treatment for Type 2 Diabetes: Structures and Inhibition of Human Intestinal Maltase-Glucoamylase with Compounds from Salacia reticulata
journal, January 2010
- Sim, Lyann; Jayakanthan, Kumarasamy; Mohan, Sankar
- Biochemistry, Vol. 49, Issue 3
An obesity-associated gut microbiome with increased capacity for energy harvest
journal, December 2006
- Turnbaugh, Peter J.; Ley, Ruth E.; Mahowald, Michael A.
- Nature, Vol. 444, Issue 7122
Structural Basis for Substrate Selectivity in Human Maltase-Glucoamylase and Sucrase-Isomaltase N-terminal Domains
journal, March 2010
- Sim, Lyann; Willemsma, Carly; Mohan, Sankar
- Journal of Biological Chemistry, Vol. 285, Issue 23
Voglibose (AO-128) Is an Efficient α-Glucosidase Inhibitor and Mobilizes the Endogenous GLP-1 Reserve
journal, January 1995
- Göke, B.; Fuder, H.; Wieckhorst, G.
- Digestion, Vol. 56, Issue 6
The Path Forward for Biofuels and Biomaterials
journal, January 2006
- Ragauskas, Arthur J.; Williams, Charlotte K.; Davison, Brian H.
- Science, Vol. 311, Issue 5760, p. 484-489
-Glucosidase Inhibition by Miglitol in NIDDM Patients
journal, April 1992
- Kingma, P. J.; Menheere, P. P.; Sels, J. P.
- Diabetes Care, Vol. 15, Issue 4
Effects of orally administered antibiotics on the bioavailability of amlodipine: gut microbiota-mediated drug interaction
journal, January 2016
- Yoo, Hye Hyun; Kim, In Sook; Yoo, Dae-Hyeong
- Journal of Hypertension, Vol. 34, Issue 1
The Carbohydrate-Active EnZymes database (CAZy): an expert resource for Glycogenomics
journal, January 2009
- Cantarel, B. L.; Coutinho, P. M.; Rancurel, C.
- Nucleic Acids Research, Vol. 37, Issue Database
Gut Microbiota-Mediated Drug Interactions between Lovastatin and Antibiotics
journal, June 2014
- Yoo, Dae-Hyoung; Kim, In Sook; Van Le, Thi Kim
- Drug Metabolism and Disposition, Vol. 42, Issue 9
Efficacy and Safety Profile Evaluation of Acarbose Alone and in Association With Other Antidiabetic Drugs: A Systematic Review
journal, June 2012
- Derosa, Giuseppe; Maffioli, Pamela
- Clinical Therapeutics, Vol. 34, Issue 6
Automation of protein purification for structural genomics
journal, March 2004
- Kim, Youngchang; Dementieva, Irina; Zhou, Min
- Journal of Structural and Functional Genomics, Vol. 5, Issue 1/2
Structure of human lysosomal acid α-glucosidase–a guide for the treatment of Pompe disease
journal, October 2017
- Roig-Zamboni, Véronique; Cobucci-Ponzano, Beatrice; Iacono, Roberta
- Nature Communications, Vol. 8, Issue 1
Alleviating Cancer Drug Toxicity by Inhibiting a Bacterial Enzyme
journal, November 2010
- Wallace, B. D.; Wang, H.; Lane, K. T.
- Science, Vol. 330, Issue 6005
The influence of gut microbiota on drug metabolism and toxicity
journal, November 2015
- Li, Houkai; He, Jiaojiao; Jia, Wei
- Expert Opinion on Drug Metabolism & Toxicology, Vol. 12, Issue 1
The NIH Human Microbiome Project
journal, October 2009
- Peterson, J.; Garges, S.; Giovanni, M.
- Genome Research, Vol. 19, Issue 12
The convergence of carbohydrate active gene repertoires in human gut microbes
journal, September 2008
- Lozupone, C. A.; Hamady, M.; Cantarel, B. L.
- Proceedings of the National Academy of Sciences, Vol. 105, Issue 39
Gut microbiota modulation with norfloxacin and ampicillin enhances glucose tolerance in mice
journal, July 2008
- Membrez, Mathieu; Blancher, Florence; Jaquet, Muriel
- The FASEB Journal, Vol. 22, Issue 7
Human Intestinal Maltase–Glucoamylase: Crystal Structure of the N-Terminal Catalytic Subunit and Basis of Inhibition and Substrate Specificity
journal, January 2008
- Sim, Lyann; Quezada-Calvillo, Roberto; Sterchi, Erwin E.
- Journal of Molecular Biology, Vol. 375, Issue 3
Improved glycaemic control with miglitol in inadequately-controlled type 2 diabetics
journal, March 2001
- Standl, E.; Schernthaner, G.; Rybka, J.
- Diabetes Research and Clinical Practice, Vol. 51, Issue 3
Metabolic Diversity of the Intestinal Microbiota: Implications for Health and Disease
journal, March 2007
- Blaut, Michael; Clavel, Thomas
- The Journal of Nutrition, Vol. 137, Issue 3
Biosynthesis of aminocyclitol-aminoglycoside antibiotics and related compounds
journal, January 2007
- Flatt, Patricia M.; Mahmud, Taifo
- Nat. Prod. Rep., Vol. 24, Issue 2
HKL -3000: the integration of data reduction and structure solution – from diffraction images to an initial model in minutes
journal, July 2006
- Minor, Wladek; Cymborowski, Marcin; Otwinowski, Zbyszek
- Acta Crystallographica Section D Biological Crystallography, Vol. 62, Issue 8
Molecular replacement with MOLREP
journal, December 2009
- Vagin, Alexei; Teplyakov, Alexei
- Acta Crystallographica Section D Biological Crystallography, Vol. 66, Issue 1
Coot model-building tools for molecular graphics
journal, November 2004
- Emsley, Paul; Cowtan, Kevin
- Acta Crystallographica Section D Biological Crystallography, Vol. 60, Issue 12, p. 2126-2132
Optimal description of a protein structure in terms of multiple groups undergoing TLS motion
journal, March 2006
- Painter, Jay; Merritt, Ethan A.
- Acta Crystallographica Section D Biological Crystallography, Vol. 62, Issue 4
Works referencing / citing this record:
Predicting associations among drugs, targets and diseases by tensor decomposition for drug repositioning
journal, December 2019
- Wang, Ran; Li, Shuai; Cheng, Lixin
- BMC Bioinformatics, Vol. 20, Issue S26
Identification of α-glucosidase inhibitors from cyclocarya paliurus tea leaves using UF-UPLC-Q/TOF-MS/MS and molecular docking
journal, January 2019
- Ning, Zi-wan; Zhai, Li-xiang; Huang, Tao
- Food & Function, Vol. 10, Issue 4
Clinical and genetic predictors of diabetes drug’s response
journal, August 2019
- Fodor, Adriana; Cozma, Angela; Suharoschi, Ramona
- Drug Metabolism Reviews, Vol. 51, Issue 4
Predicting associations among drugs, targets and diseases by tensor decomposition for drug repositioning
journal, December 2019
- Wang, Ran; Li, Shuai; Cheng, Lixin
- BMC Bioinformatics, Vol. 20, Issue S26
Crosstalk between gut microbiota and antidiabetic drug action
journal, March 2019
- Kyriachenko, Yevheniia; Falalyeyeva, Tetyana; Korotkyi, Oleksandr
- World Journal of Diabetes, Vol. 10, Issue 3
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal