Discovery and Characterization of a Thioesterase-Specific Monoclonal Antibody That Recognizes the 6-Deoxyerythronolide B Synthase
Abstract
Assembly line polyketide synthases (PKSs) are large multimodular enzymes responsible for the biosynthesis of diverse antibiotics in bacteria. Structural and mechanistic analysis of these megasynthases can benefit from the discovery of reagents that recognize individual domains or linkers in a site-specific manner. Monoclonal antibodies not only have proven themselves as premier tools in analogous applications but also have the added benefit of constraining the conformational flexibility of their targets in unpredictable but often useful ways. Here we have exploited a library based on the naïve human antibody repertoire to discover a Fab (3A6) that recognizes the terminal thioesterase (TE) domain of the 6-deoxyerythronolide B synthase with high specificity. Biochemical assays were used to verify that 3A6 binding does not inhibit enzyme turnover. The co-crystal structure of the TE–3A6 complex was determined at 2.45 Å resolution, resulting in atomic characterization of this protein–protein recognition mechanism. Fab binding had minimal effects on the structural integrity of the TE. In turn, these insights were used to interrogate via small-angle X-ray scattering the solution-phase conformation of 3A6 complexed to a catalytically competent PKS module and bimodule. Furthermore, we have developed a high-affinity monoclonal antibody tool that recognizes the TE domain of the 6-deoxyerythronolide Bmore »
- Authors:
-
- Stanford Univ., Stanford, CA (United States)
- Univ. of California San Francisco, San Francisco, CA (United States)
- Stanford Univ., Menlo Park, CA (United States)
- Publication Date:
- Research Org.:
- SLAC National Accelerator Lab., Menlo Park, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Biological and Environmental Research (BER); USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1490870
- Grant/Contract Number:
- P41CA196276; AC02-76SF00515; GM087934, GM104659, P41GM103393
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Biochemistry
- Additional Journal Information:
- Journal Volume: 57; Journal Issue: 43; Journal ID: ISSN 0006-2960
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; 59 BASIC BIOLOGICAL SCIENCES
Citation Formats
Li, Xiuyuan, Sevillano, Natalia, La Greca, Florencia, Hsu, Jake, Mathews, Irimpan I., Matsui, Tsutomu, Craik, Charles S., and Khosla, Chaitan. Discovery and Characterization of a Thioesterase-Specific Monoclonal Antibody That Recognizes the 6-Deoxyerythronolide B Synthase. United States: N. p., 2018.
Web. doi:10.1021/acs.biochem.8b00886.
Li, Xiuyuan, Sevillano, Natalia, La Greca, Florencia, Hsu, Jake, Mathews, Irimpan I., Matsui, Tsutomu, Craik, Charles S., & Khosla, Chaitan. Discovery and Characterization of a Thioesterase-Specific Monoclonal Antibody That Recognizes the 6-Deoxyerythronolide B Synthase. United States. https://doi.org/10.1021/acs.biochem.8b00886
Li, Xiuyuan, Sevillano, Natalia, La Greca, Florencia, Hsu, Jake, Mathews, Irimpan I., Matsui, Tsutomu, Craik, Charles S., and Khosla, Chaitan. Fri .
"Discovery and Characterization of a Thioesterase-Specific Monoclonal Antibody That Recognizes the 6-Deoxyerythronolide B Synthase". United States. https://doi.org/10.1021/acs.biochem.8b00886. https://www.osti.gov/servlets/purl/1490870.
@article{osti_1490870,
title = {Discovery and Characterization of a Thioesterase-Specific Monoclonal Antibody That Recognizes the 6-Deoxyerythronolide B Synthase},
author = {Li, Xiuyuan and Sevillano, Natalia and La Greca, Florencia and Hsu, Jake and Mathews, Irimpan I. and Matsui, Tsutomu and Craik, Charles S. and Khosla, Chaitan},
abstractNote = {Assembly line polyketide synthases (PKSs) are large multimodular enzymes responsible for the biosynthesis of diverse antibiotics in bacteria. Structural and mechanistic analysis of these megasynthases can benefit from the discovery of reagents that recognize individual domains or linkers in a site-specific manner. Monoclonal antibodies not only have proven themselves as premier tools in analogous applications but also have the added benefit of constraining the conformational flexibility of their targets in unpredictable but often useful ways. Here we have exploited a library based on the naïve human antibody repertoire to discover a Fab (3A6) that recognizes the terminal thioesterase (TE) domain of the 6-deoxyerythronolide B synthase with high specificity. Biochemical assays were used to verify that 3A6 binding does not inhibit enzyme turnover. The co-crystal structure of the TE–3A6 complex was determined at 2.45 Å resolution, resulting in atomic characterization of this protein–protein recognition mechanism. Fab binding had minimal effects on the structural integrity of the TE. In turn, these insights were used to interrogate via small-angle X-ray scattering the solution-phase conformation of 3A6 complexed to a catalytically competent PKS module and bimodule. Furthermore, we have developed a high-affinity monoclonal antibody tool that recognizes the TE domain of the 6-deoxyerythronolide B synthase while maintaining its native function.},
doi = {10.1021/acs.biochem.8b00886},
journal = {Biochemistry},
number = 43,
volume = 57,
place = {United States},
year = {Fri Oct 05 00:00:00 EDT 2018},
month = {Fri Oct 05 00:00:00 EDT 2018}
}
Web of Science
Figures / Tables:
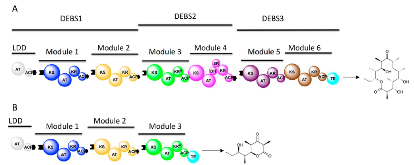 Figure 1: (A) Synthesis of 6-deoxyerythronolide B by DEBS. Abbreviations: ACP, acyl carrier protein; AT, acyltransferase; DH, dehydratase; KR, ketoreductase; KR0, inactive ketoreductase; KS, ketosynthase. Whereas the loading didomain (LDD) and the first two modules of DEBS occur within a single protein (DEBS!) in nature, our in vitro reconstituted systemmore »
Figure 1: (A) Synthesis of 6-deoxyerythronolide B by DEBS. Abbreviations: ACP, acyl carrier protein; AT, acyltransferase; DH, dehydratase; KR, ketoreductase; KR0, inactive ketoreductase; KS, ketosynthase. Whereas the loading didomain (LDD) and the first two modules of DEBS occur within a single protein (DEBS!) in nature, our in vitro reconstituted systemmore »
Works referenced in this record:
Polyketide and Nonribosomal Peptide Antibiotics: Modularity and Versatility
journal, March 2004
- Walsh, C. T.
- Science, Vol. 303, Issue 5665
Structure and Mechanism of the 6-Deoxyerythronolide B Synthase
journal, June 2007
- Khosla, Chaitan; Tang, Yinyan; Chen, Alice Y.
- Annual Review of Biochemistry, Vol. 76, Issue 1
An unusually large multifunctional polypeptide in the erythromycin-producing polyketide synthase of Saccharopolyspora erythraea
journal, November 1990
- Cortes, Jesus; Haydock, Stephen F.; Roberts, Gareth A.
- Nature, Vol. 348, Issue 6297
Modular organization of genes required for complex polyketide biosynthesis
journal, May 1991
- Donadio, S.; Staver, M.; McAlpine, J.
- Science, Vol. 252, Issue 5006
Polyketide synthase and non-ribosomal peptide synthetase thioesterase selectivity: logic gate or a victim of fate?
journal, January 2016
- Horsman, Mark E.; Hari, Taylor P. A.; Boddy, Christopher N.
- Natural Product Reports, Vol. 33, Issue 2
In Vitro Reconstitution and Analysis of the 6-Deoxyerythronolide B Synthase
journal, October 2013
- Lowry, Brian; Robbins, Thomas; Weng, Chih-Hisang
- Journal of the American Chemical Society, Vol. 135, Issue 45
Crystallization of a challenging antigen–antibody complex: TLR3 ECD with three noncompeting Fabs
journal, September 2011
- Malia, Thomas J.; Obmolova, Galina; Luo, Jinquan
- Acta Crystallographica Section F Structural Biology and Crystallization Communications, Vol. 67, Issue 10
Chemistry of ion coordination and hydration revealed by a K+ channel–Fab complex at 2.0 Å resolution
journal, November 2001
- Zhou, Yufeng; Morais-Cabral, João H.; Kaufman, Amelia
- Nature, Vol. 414, Issue 6859
Crystal structure of full-length KcsA in its closed conformation
journal, April 2009
- Uysal, S.; Vasquez, V.; Tereshko, V.
- Proceedings of the National Academy of Sciences, Vol. 106, Issue 16
Structure of an Fab–Protease Complex Reveals a Highly Specific Non-canonical Mechanism of Inhibition
journal, July 2008
- Farady, Christopher J.; Egea, Pascal F.; Schneider, Eric L.
- Journal of Molecular Biology, Vol. 380, Issue 2
Structure–Function Analysis of the Extended Conformation of a Polyketide Synthase Module
journal, May 2018
- Li, Xiuyuan; Sevillano, Natalia; La Greca, Florencia
- Journal of the American Chemical Society, Vol. 140, Issue 21
Architectures of Whole-Module and Bimodular Proteins from the 6-Deoxyerythronolide B Synthase
journal, May 2014
- Edwards, Andrea L.; Matsui, Tsutomu; Weiss, Thomas M.
- Journal of Molecular Biology, Vol. 426, Issue 11
Quantitative Analysis of the Relative Contributions of Donor Acyl Carrier Proteins, Acceptor Ketosynthases, and Linker Regions to Intermodular Transfer of Intermediates in Hybrid Polyketide Synthases †
journal, April 2002
- Wu, Nicholas; Cane, David E.; Khosla, Chaitan
- Biochemistry, Vol. 41, Issue 15
Extender Unit and Acyl Carrier Protein Specificity of Ketosynthase Domains of the 6-Deoxyerythronolide B Synthase
journal, March 2006
- Chen, Alice Y.; Schnarr, Nathan A.; Kim, Chu-Young
- Journal of the American Chemical Society, Vol. 128, Issue 9
Antagonistic Anti-urokinase Plasminogen Activator Receptor (uPAR) Antibodies Significantly Inhibit uPAR-mediated Cellular Signaling and Migration
journal, August 2010
- Duriseti, Sai; Goetz, David H.; Hostetter, Daniel R.
- Journal of Biological Chemistry, Vol. 285, Issue 35
Rapid identification of recombinant Fabs that bind to membrane proteins
journal, December 2011
- Kim, JungMin; Stroud, Robert M.; Craik, Charles S.
- Methods, Vol. 55, Issue 4
Subnanometre-resolution electron cryomicroscopy structure of a heterodimeric ABC exporter
journal, November 2014
- Kim, JungMin; Wu, Shenping; Tomasiak, Thomas M.
- Nature, Vol. 517, Issue 7534
Enzymatic Extender Unit Generation for In Vitro Polyketide Synthase Reactions: Structural and Func-tional Showcasing of Streptomyces coelicolor MatB
journal, February 2011
- Hughes, Amanda J.; Keatinge-Clay, Adrian
- Chemistry & Biology, Vol. 18, Issue 2
XDS
journal, January 2010
- Kabsch, Wolfgang
- Acta Crystallographica Section D Biological Crystallography, Vol. 66, Issue 2
MOLREP an Automated Program for Molecular Replacement
journal, December 1997
- Vagin, A.; Teplyakov, A.
- Journal of Applied Crystallography, Vol. 30, Issue 6, p. 1022-1025
Insights into Channel Architecture and Substrate Specificity from Crystal Structures of Two Macrocycle-Forming Thioesterases of Modular Polyketide Synthases † , ‡
journal, October 2002
- Tsai, Shiou-Chuan; Lu, Hongxiang; Cane, David E.
- Biochemistry, Vol. 41, Issue 42
Mechanism for neutralizing activity by the anti-CMV gH/gL monoclonal antibody MSL-109
journal, May 2014
- Fouts, A. E.; Comps-Agrar, L.; Stengel, K. F.
- Proceedings of the National Academy of Sciences, Vol. 111, Issue 22
A knowledge-driven approach for crystallographic protein model completion
journal, March 2008
- Joosten, Krista; Cohen, Serge X.; Emsley, Paul
- Acta Crystallographica Section D Biological Crystallography, Vol. 64, Issue 4
The Buccaneer software for automated model building. 1. Tracing protein chains
journal, August 2006
- Cowtan, Kevin
- Acta Crystallographica Section D Biological Crystallography, Vol. 62, Issue 9
Features and development of Coot
journal, March 2010
- Emsley, P.; Lohkamp, B.; Scott, W. G.
- Acta Crystallographica Section D Biological Crystallography, Vol. 66, Issue 4
PHENIX: a comprehensive Python-based system for macromolecular structure solution
journal, January 2010
- Adams, Paul D.; Afonine, Pavel V.; Bunkóczi, Gábor
- Acta Crystallographica Section D Biological Crystallography, Vol. 66, Issue 2, p. 213-221
MolProbity : all-atom structure validation for macromolecular crystallography
journal, December 2009
- Chen, Vincent B.; Arendall, W. Bryan; Headd, Jeffrey J.
- Acta Crystallographica Section D Biological Crystallography, Vol. 66, Issue 1
CRYSOL – a Program to Evaluate X-ray Solution Scattering of Biological Macromolecules from Atomic Coordinates
journal, December 1995
- Svergun, D.; Barberato, C.; Koch, M. H. J.
- Journal of Applied Crystallography, Vol. 28, Issue 6
Crystal structure of the macrocycle-forming thioesterase domain of the erythromycin polyketide synthase: Versatility from a unique substrate channel
journal, December 2001
- Tsai, S. -C.; Miercke, L. J. W.; Krucinski, J.
- Proceedings of the National Academy of Sciences, Vol. 98, Issue 26
Towards a characterization of the structural determinants of specificity in the macrocyclizing thioesterase for deoxyerythronolide B biosynthesis
journal, March 2016
- Argyropoulos, Panos; Bergeret, Fabien; Pardin, Christophe
- Biochimica et Biophysica Acta (BBA) - General Subjects, Vol. 1860, Issue 3
Fabs Enable Single Particle cryoEM Studies of Small Proteins
journal, April 2012
- Wu, Shenping; Avila-Sakar, Agustin; Kim, JungMin
- Structure, Vol. 20, Issue 4
Works referencing / citing this record:
Why does tautomycetin thioesterase prefer hydrolysis to macrocyclization? Theoretical study on its catalytic mechanism
journal, January 2019
- Liu, Lei; Tao, Wentao; Bai, Linquan
- Catalysis Science & Technology, Vol. 9, Issue 22

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal