Direct observation of the kinetics of gas–solid reactions using in situ kinetic and spectroscopic techniques
Abstract
Developing fundamental insight for reactions between gas phase H2S and solid phase CuO has the potential to lead to improved materials and processes for natural gas purification. However, this insight requires detailed knowledge of the atomistic characteristics of the solid and how these characteristics influence the reaction mechanism and kinetics. Herein, we use fixed bed reactors, X-ray absorption spectroscopy, and transmission X-ray microscopy to simultaneously probe the fundamental kinetics of the reaction of CuO with H2S to form CuS, and thereby probe spatial-temporal chemical and structural changes of copper during this reaction. H2S removal reaction kinetics show similar trends in fixed bed reactors as in 10-20 µm sized particles. However, reaction fronts proceed through the entire diameter of particles heterogeneously, indicating the presence of pore diffusion resistance even at very small length scales. In addition, CuO sorbent samples with similar characteristics exhibit 3 times different sulfidation conversion with reaction rate constants that differ by a factor of 1.5. Furthermore, these differences in reaction kinetics and conversion indicate the critical impact of possible atomic scale differences and the formation of different copper sulfide products.
- Authors:
-
- SLAC National Accelerator Lab., Menlo Park, CA (United States)
- Univ. of California, Los Angeles, CA (United States)
- SLAC National Accelerator Lab., Menlo Park, CA (United States); Chinese Academy of Sciences (CAS), Beijing (China)
- Publication Date:
- Research Org.:
- SLAC National Accelerator Lab., Menlo Park, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES)
- OSTI Identifier:
- 1484837
- Alternate Identifier(s):
- OSTI ID: 1458581
- Grant/Contract Number:
- 11535015; U1632110; 56978-DNI5; AC02-76SF00515
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Reaction Chemistry & Engineering
- Additional Journal Information:
- Journal Volume: 3; Journal Issue: 5; Journal ID: ISSN 2058-9883
- Publisher:
- Royal Society of Chemistry
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Hoffman, Adam S., Azzam, Sara, Zhang, Kai, Xu, Yahong, Liu, Yijin, Bare, Simon R., and Simonetti, Dante A. Direct observation of the kinetics of gas–solid reactions using in situ kinetic and spectroscopic techniques. United States: N. p., 2018.
Web. doi:10.1039/c8re00020d.
Hoffman, Adam S., Azzam, Sara, Zhang, Kai, Xu, Yahong, Liu, Yijin, Bare, Simon R., & Simonetti, Dante A. Direct observation of the kinetics of gas–solid reactions using in situ kinetic and spectroscopic techniques. United States. https://doi.org/10.1039/c8re00020d
Hoffman, Adam S., Azzam, Sara, Zhang, Kai, Xu, Yahong, Liu, Yijin, Bare, Simon R., and Simonetti, Dante A. Thu .
"Direct observation of the kinetics of gas–solid reactions using in situ kinetic and spectroscopic techniques". United States. https://doi.org/10.1039/c8re00020d. https://www.osti.gov/servlets/purl/1484837.
@article{osti_1484837,
title = {Direct observation of the kinetics of gas–solid reactions using in situ kinetic and spectroscopic techniques},
author = {Hoffman, Adam S. and Azzam, Sara and Zhang, Kai and Xu, Yahong and Liu, Yijin and Bare, Simon R. and Simonetti, Dante A.},
abstractNote = {Developing fundamental insight for reactions between gas phase H2S and solid phase CuO has the potential to lead to improved materials and processes for natural gas purification. However, this insight requires detailed knowledge of the atomistic characteristics of the solid and how these characteristics influence the reaction mechanism and kinetics. Herein, we use fixed bed reactors, X-ray absorption spectroscopy, and transmission X-ray microscopy to simultaneously probe the fundamental kinetics of the reaction of CuO with H2S to form CuS, and thereby probe spatial-temporal chemical and structural changes of copper during this reaction. H2S removal reaction kinetics show similar trends in fixed bed reactors as in 10-20 µm sized particles. However, reaction fronts proceed through the entire diameter of particles heterogeneously, indicating the presence of pore diffusion resistance even at very small length scales. In addition, CuO sorbent samples with similar characteristics exhibit 3 times different sulfidation conversion with reaction rate constants that differ by a factor of 1.5. Furthermore, these differences in reaction kinetics and conversion indicate the critical impact of possible atomic scale differences and the formation of different copper sulfide products.},
doi = {10.1039/c8re00020d},
journal = {Reaction Chemistry & Engineering},
number = 5,
volume = 3,
place = {United States},
year = {Thu Jun 21 00:00:00 EDT 2018},
month = {Thu Jun 21 00:00:00 EDT 2018}
}
Figures / Tables:
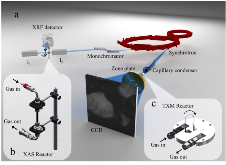 Figure 1: a) Schematic of experimental setup for sulfidation of CuO samples at the XAS and TXM beam lines. b) XAS cell schematic used for the room temperature sulfidation of CuO. c) TXM cell schematic featuring 0.5 mm quartz capillary used for the room temperature sulfidation of CuO.
Figure 1: a) Schematic of experimental setup for sulfidation of CuO samples at the XAS and TXM beam lines. b) XAS cell schematic used for the room temperature sulfidation of CuO. c) TXM cell schematic featuring 0.5 mm quartz capillary used for the room temperature sulfidation of CuO.
Works referenced in this record:
Synchrotron X-ray Analytical Techniques for Studying Materials Electrochemistry in Rechargeable Batteries
journal, September 2017
- Lin, Feng; Liu, Yijin; Yu, Xiqian
- Chemical Reviews, Vol. 117, Issue 21
The Reaction between Hydrogen Sulfide and Spherical Pellets of Zinc Oxide
journal, April 1980
- Gibson, James B.; Harrison, Douglas P.
- Industrial & Engineering Chemistry Process Design and Development, Vol. 19, Issue 2
Three-dimensional imaging of chemical phase transformations at the nanoscale with full-field transmission X-ray microscopy
journal, July 2011
- Meirer, Florian; Cabana, Jordi; Liu, Yijin
- Journal of Synchrotron Radiation, Vol. 18, Issue 5
Hard X-ray Nanotomography of Catalytic Solids at Work
journal, October 2012
- Gonzalez-Jimenez, Ines D.; Cats, Korneel; Davidian, Thomas
- Angewandte Chemie International Edition, Vol. 51, Issue 48
Strategies for the Direct Catalytic Valorization of Methane Using Heterogeneous Catalysis: Challenges and Opportunities
journal, April 2016
- Olivos-Suarez, Alma I.; Szécsényi, Àgnes; Hensen, Emiel J. M.
- ACS Catalysis, Vol. 6, Issue 5
New design approaches to ultra-clean diesel fuels by deep desulfurization and deep dearomatization
journal, March 2003
- Song, Chunshan; Ma, Xiaoliang
- Applied Catalysis B: Environmental, Vol. 41, Issue 1-2
A random pore model for fluid-solid reactions: I. Isothermal, kinetic control
journal, May 1980
- Bhatia, S. K.; Perlmutter, D. D.
- AIChE Journal, Vol. 26, Issue 3
Review of Experimental Characterization of Active Sites and Determination of Molecular Mechanisms of Adsorption, Desorption and Regeneration of the Deep and Ultradeep Desulfurization Sorbents for Liquid Fuels
journal, July 2010
- Samokhvalov, Alexander; Tatarchuk, Bruce J.
- Catalysis Reviews, Vol. 52, Issue 3
Correlating Metal Poisoning with Zeolite Deactivation in an Individual Catalyst Particle by Chemical and Phase-Sensitive X-ray Microscopy
journal, April 2013
- Ruiz-Martínez, Javier; Beale, Andrew M.; Deka, Upakul
- Angewandte Chemie International Edition, Vol. 52, Issue 23
Characterization of active sites, determination of mechanisms of H2S, COS and CS2 sorption and regeneration of ZnO low-temperature sorbents: past, current and perspectives
journal, January 2011
- Samokhvalov, Alexander; Tatarchuk, Bruce J.
- Physical Chemistry Chemical Physics, Vol. 13, Issue 8
Modeling of noncatalytic gas-solid reactions
journal, November 1982
- Ramachandran, P. A.; Doraiswamy, L. K.
- AIChE Journal, Vol. 28, Issue 6
TXM-Wizard : a program for advanced data collection and evaluation in full-field transmission X-ray microscopy
journal, January 2012
- Liu, Yijin; Meirer, Florian; Williams, Phillip A.
- Journal of Synchrotron Radiation, Vol. 19, Issue 2
Sulfidation of zinc titanate and zinc oxide solids
journal, August 1992
- Lew, Susan; Sarofim, Adel F.; Flytzani-Stephanopoulos, Maria
- Industrial & Engineering Chemistry Research, Vol. 31, Issue 8
Regenerative Adsorption and Removal of H2S from Hot Fuel Gas Streams by Rare Earth Oxides
journal, June 2006
- Flytzani-Stephanopoulos, M.
- Science, Vol. 312, Issue 5779
XPS, XANES and EXAFS investigations of CuO/ZnO/Al2O3/ZrO2 mixed oxide catalysts
journal, April 2002
- Velu, S.; Suzuki, K.; Gopinath, Chinnakonda S.
- Physical Chemistry Chemical Physics, Vol. 4, Issue 10
New insight into the ZnO sulfidation reaction: mechanism and kinetics modeling of the ZnS outward growth
journal, January 2013
- Neveux, Laure; Chiche, David; Pérez-Pellitero, Javier
- Phys. Chem. Chem. Phys., Vol. 15, Issue 5
EXAFS and XANES analysis of oxides at the nanoscale
journal, October 2014
- Kuzmin, Alexei; Chaboy, Jesús
- IUCrJ, Vol. 1, Issue 6
Contributions of magnetic properties in epitaxial copper-doped ZnO
journal, January 2013
- Liu, Hongyan; Zeng, Fei; Gao, Shuang
- Physical Chemistry Chemical Physics, Vol. 15, Issue 31
Hard X-ray Nanotomography of Catalytic Solids at Work
journal, October 2012
- Gonzalez-Jimenez, Ines D.; Cats, Korneel; Davidian, Thomas
- Angewandte Chemie, Vol. 124, Issue 48
Correlating Metal Poisoning with Zeolite Deactivation in an Individual Catalyst Particle by Chemical and Phase-Sensitive X-ray Microscopy
journal, April 2013
- Ruiz-Martínez, Javier; Beale, Andrew M.; Deka, Upakul
- Angewandte Chemie, Vol. 125, Issue 23
Regenerative Adsorption and Removal of H2S from Hot Fuel Gas Streams by Rare Earth Oxides.
journal, September 2006
- Flytzani-Stephanopoulos, Maria; Sakbodin, Mann; Wang, Zheng
- ChemInform, Vol. 37, Issue 36
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal