1,2-Addition of Formic or Oxalic Acid to –N{CH2CH2(PiPr2)}2 -Supported Mn(I) Dicarbonyl Complexes and the Manganese-Mediated Decomposition of Formic Acid
Abstract
(PNHP)Mn(CO)2 (I) carboxylate complexes (PNHP = HN{CH2CH2(PiPr2)}2) were prepared in this study via 1,2-addition of either formic or oxalic acid to (PNP)Mn(CO)2 (PNP = the deprotonated, amide form of the ligand –N{CH2CH2(PiPr2)}2). The structural and spectral properties of these complexes were compared. The manganese formate complex was found to be dimeric in the solid state and monomeric in solution. Half an equivalent of oxalic acid was employed to form the bridging oxalate dimanganese complex. The catalytic competencies of the carboxylate complexes were assessed, and the formate complex was found to decompose formic acid catalytically. Both dehydrogenation and dehydration pathways were active as assessed by the presence of H2, CO2, and H2O. Lastly, the addition of LiBF4 exhibited a strong inhibitory effect on the catalysis.
- Authors:
-
- Los Alamos National Lab. (LANL), Los Alamos, NM (United States)
- Publication Date:
- Research Org.:
- Los Alamos National Laboratory (LANL), Los Alamos, NM (United States)
- Sponsoring Org.:
- USDOE Laboratory Directed Research and Development (LDRD) Program
- OSTI Identifier:
- 1483496
- Report Number(s):
- LA-UR-16-22530
Journal ID: ISSN 0276-7333
- Grant/Contract Number:
- 89233218CNA000001
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Organometallics
- Additional Journal Information:
- Journal Volume: 35; Journal Issue: 12; Journal ID: ISSN 0276-7333
- Publisher:
- American Chemical Society
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Tondreau, Aaron M., and Boncella, James M. 1,2-Addition of Formic or Oxalic Acid to –N{CH2CH2(PiPr2)}2 -Supported Mn(I) Dicarbonyl Complexes and the Manganese-Mediated Decomposition of Formic Acid. United States: N. p., 2016.
Web. doi:10.1021/acs.organomet.6b00274.
Tondreau, Aaron M., & Boncella, James M. 1,2-Addition of Formic or Oxalic Acid to –N{CH2CH2(PiPr2)}2 -Supported Mn(I) Dicarbonyl Complexes and the Manganese-Mediated Decomposition of Formic Acid. United States. https://doi.org/10.1021/acs.organomet.6b00274
Tondreau, Aaron M., and Boncella, James M. Tue .
"1,2-Addition of Formic or Oxalic Acid to –N{CH2CH2(PiPr2)}2 -Supported Mn(I) Dicarbonyl Complexes and the Manganese-Mediated Decomposition of Formic Acid". United States. https://doi.org/10.1021/acs.organomet.6b00274. https://www.osti.gov/servlets/purl/1483496.
@article{osti_1483496,
title = {1,2-Addition of Formic or Oxalic Acid to –N{CH2CH2(PiPr2)}2 -Supported Mn(I) Dicarbonyl Complexes and the Manganese-Mediated Decomposition of Formic Acid},
author = {Tondreau, Aaron M. and Boncella, James M.},
abstractNote = {(PNHP)Mn(CO)2 (I) carboxylate complexes (PNHP = HN{CH2CH2(PiPr2)}2) were prepared in this study via 1,2-addition of either formic or oxalic acid to (PNP)Mn(CO)2 (PNP = the deprotonated, amide form of the ligand –N{CH2CH2(PiPr2)}2). The structural and spectral properties of these complexes were compared. The manganese formate complex was found to be dimeric in the solid state and monomeric in solution. Half an equivalent of oxalic acid was employed to form the bridging oxalate dimanganese complex. The catalytic competencies of the carboxylate complexes were assessed, and the formate complex was found to decompose formic acid catalytically. Both dehydrogenation and dehydration pathways were active as assessed by the presence of H2, CO2, and H2O. Lastly, the addition of LiBF4 exhibited a strong inhibitory effect on the catalysis.},
doi = {10.1021/acs.organomet.6b00274},
journal = {Organometallics},
number = 12,
volume = 35,
place = {United States},
year = {Tue Jun 14 00:00:00 EDT 2016},
month = {Tue Jun 14 00:00:00 EDT 2016}
}
Web of Science
Figures / Tables:
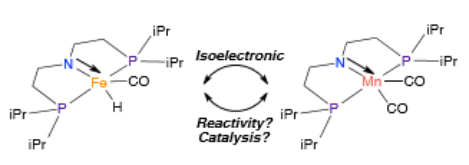 Figure 1: Comparison of isoelectronic metal complexes of Fe(II) and Mn(I).
Figure 1: Comparison of isoelectronic metal complexes of Fe(II) and Mn(I).
Works referenced in this record:
Breakthroughs in Hydrogen Storage-Formic Acid as a Sustainable Storage Material for Hydrogen
journal, October 2008
- Joó, Ferenc
- ChemSusChem, Vol. 1, Issue 10
Hydrogen generation from formic acid and alcohols using homogeneous catalysts
journal, January 2010
- Johnson, Tarn C.; Morris, David J.; Wills, Martin
- Chem. Soc. Rev., Vol. 39, Issue 1
Catalytic Generation of Hydrogen from Formic acid and its Derivatives: Useful Hydrogen Storage Materials
journal, May 2010
- Loges, Björn; Boddien, Albert; Gärtner, Felix
- Topics in Catalysis, Vol. 53, Issue 13-14
Homogeneous catalysis by ruthenium carbonyl in alkaline solution: the water gas shift reaction
journal, January 1977
- Laine, Richard M.; Rinker, Robert G.; Ford, Peter C.
- Journal of the American Chemical Society, Vol. 99, Issue 1
An efficient binuclear catalyst for decomposition of formic acid
journal, January 1998
- Gao, Yuan; Kuncheria, Joshi; Puddephatt, Richard J.
- Chemical Communications, Issue 21
The interconversion of formic acid and hydrogen/carbon dioxide using a binuclear ruthenium complex catalyst
journal, January 2000
- Gao, Yuan; Kuncheria, Joshi K.; Jenkins, Hilary A.
- Journal of the Chemical Society, Dalton Transactions, Issue 18
Synthesis, characterization and reactivity of heterobimetallic complexes (η 5 -C 5 R 5 )Ru(CO)(μ-dppm)M(CO) 2 (η 5 -C 5 H 5 ) (R = H, CH 3 ; M = Mo, W). Interconversion of hydrogen/carbon dioxide and formic acid by these complexes
journal, January 2003
- Man, Man Lok; Zhou, Zhongyuan; Ng, Siu Man
- Dalton Trans., Issue 19
Controlled Generation of Hydrogen from Formic Acid Amine Adducts at Room Temperature and Application in H 2 /O 2 Fuel Cells
journal, May 2008
- Loges, Björn; Boddien, Albert; Junge, Henrik
- Angewandte Chemie International Edition, Vol. 47, Issue 21
A Viable Hydrogen-Storage System Based On Selective Formic Acid Decomposition with a Ruthenium Catalyst
journal, May 2008
- Fellay, Céline; Dyson, Paul J.; Laurenczy, Gábor
- Angewandte Chemie International Edition, Vol. 47, Issue 21, p. 3966-3968
Selective Formic Acid Decomposition for High-Pressure Hydrogen Generation: A Mechanistic Study
journal, February 2009
- Fellay, Céline; Yan, Ning; Dyson, Paul J.
- Chemistry - A European Journal, Vol. 15, Issue 15
Hydrogen storage and delivery: immobilization of a highly active homogeneous catalyst for the decomposition of formic acid to hydrogen and carbon dioxide
journal, November 2009
- Gan, Weijia; Dyson, Paul J.; Laurenczy, Gábor
- Reaction Kinetics and Catalysis Letters, Vol. 98, Issue 2
Insights into Hydrogen Generation from Formic Acid Using Ruthenium Complexes
journal, July 2009
- Morris, David J.; Clarkson, Guy J.; Wills, Martin
- Organometallics, Vol. 28, Issue 14
Formic acid dehydrogenation catalysed by ruthenium complexes bearing the tripodal ligands triphos and NP 3
journal, January 2013
- Mellone, Irene; Peruzzini, Maurizio; Rosi, Luca
- Dalton Trans., Vol. 42, Issue 7
Rhodium(I) catalyzed decomposition of formic acid
journal, August 1979
- Strauss, S. H.; Whitmire, K. H.; Shriver, D. F.
- Journal of Organometallic Chemistry, Vol. 174, Issue 3
Catalytic reactions of formate 4. A nitrite-promoted rhodium (III) catalyst for hydrogen generation from formic acid in aqueous solution
journal, September 1995
- King, R. B.; Bhattacharyya, N. K.
- Inorganica Chimica Acta, Vol. 237, Issue 1-2
Efficient Catalytic Decomposition of Formic Acid for the Selective Generation of H 2 and H/D Exchange with a Water-Soluble Rhodium Complex in Aqueous Solution
journal, October 2008
- Fukuzumi, Shunichi; Kobayashi, Takeshi; Suenobu, Tomoyoshi
- ChemSusChem, Vol. 1, Issue 10
Unusually Large Tunneling Effect on Highly Efficient Generation of Hydrogen and Hydrogen Isotopes in pH-Selective Decomposition of Formic Acid Catalyzed by a Heterodinuclear Iridium−Ruthenium Complex in Water
journal, February 2010
- Fukuzumi, Shunichi; Kobayashi, Takeshi; Suenobu, Tomoyoshi
- Journal of the American Chemical Society, Vol. 132, Issue 5
Highly efficient hydrogen evolution by decomposition of formic acid using an iridium catalyst with 4,4′-dihydroxy-2,2′-bipyridine
journal, January 2009
- Himeda, Yuichiro
- Green Chemistry, Vol. 11, Issue 12
Efficient H 2 generation from formic acid using azole complexes in water
journal, January 2014
- Manaka, Yuichi; Wang, Wan-Hui; Suna, Yuki
- Catal. Sci. Technol., Vol. 4, Issue 1
Enhanced Hydrogen Generation from Formic Acid by Half-Sandwich Iridium(III) Complexes with Metal/NH Bifunctionality: A Pronounced Switch from Transfer Hydrogenation
journal, August 2015
- Matsunami, Asuka; Kayaki, Yoshihito; Ikariya, Takao
- Chemistry - A European Journal, Vol. 21, Issue 39
Unprecedentedly High Formic Acid Dehydrogenation Activity on an Iridium Complex with an N , N ′-Diimine Ligand in Water
journal, July 2015
- Wang, Zhijun; Lu, Sheng-Mei; Li, Jun
- Chemistry - A European Journal, Vol. 21, Issue 36
Reversible hydrogen storage using CO2 and a proton-switchable iridium catalyst in aqueous media under mild temperatures and pressures
journal, March 2012
- Hull, Jonathan F.; Himeda, Yuichiro; Wang, Wan-Hui
- Nature Chemistry, Vol. 4, Issue 5, p. 383-388
Long-range metal–ligand bifunctional catalysis: cyclometallated iridium catalysts for the mild and rapid dehydrogenation of formic acid
journal, January 2013
- Barnard, Jonathan H.; Wang, Chao; Berry, Neil G.
- Chemical Science, Vol. 4, Issue 3
Base-Free Production of H 2 by Dehydrogenation of Formic Acid Using An Iridium-bisMETAMORPhos Complex
journal, July 2013
- Oldenhof, Sander; de Bruin, Bas; Lutz, Martin
- Chemistry - A European Journal, Vol. 19, Issue 35
Iron-Catalyzed Hydrogen Production from Formic Acid
journal, July 2010
- Boddien, Albert; Loges, Björn; Gärtner, Felix
- Journal of the American Chemical Society, Vol. 132, Issue 26
Efficient Dehydrogenation of Formic Acid Using an Iron Catalyst
journal, September 2011
- Boddien, A.; Mellmann, D.; Gartner, F.
- Science, Vol. 333, Issue 6050
Efficient Hydrogen Liberation from Formic Acid Catalyzed by a Well-Defined Iron Pincer Complex under Mild Conditions
journal, May 2013
- Zell, Thomas; Butschke, Burkhard; Ben-David, Yehoshoa
- Chemistry - A European Journal, Vol. 19, Issue 25
Base‐Free Non‐Noble‐Metal‐Catalyzed Hydrogen Generation from Formic Acid: Scope and Mechanistic Insights
journal, September 2014
- Mellmann, Dörthe; Barsch, Enrico; Bauer, Matthias
- Chemistry – A European Journal, Vol. 20, Issue 42
Towards a Practical Setup for Hydrogen Production from Formic Acid
journal, June 2013
- Sponholz, Peter; Mellmann, Dörthe; Junge, Henrik
- ChemSusChem, Vol. 6, Issue 7
ortho-Metalation of Iron(0) Tribenzylphosphine Complexes: Homogeneous Catalysts for the Generation of Hydrogen from Formic Acid
journal, October 2010
- Boddien, Albert; Gärtner, Felix; Jackstell, Ralf
- Angewandte Chemie International Edition, Vol. 49, Issue 47
Osmium and Ruthenium Catalysts for Dehydrogenation of Alcohols
journal, July 2011
- Bertoli, Marcello; Choualeb, Aldjia; Lough, Alan J.
- Organometallics, Vol. 30, Issue 13
Selective Hydrogen Production from Methanol with a Defined Iron Pincer Catalyst under Mild Conditions
journal, December 2013
- Alberico, Elisabetta; Sponholz, Peter; Cordes, Christoph
- Angewandte Chemie International Edition, Vol. 52, Issue 52
Low-temperature aqueous-phase methanol dehydrogenation to hydrogen and carbon dioxide
journal, February 2013
- Nielsen, Martin; Alberico, Elisabetta; Baumann, Wolfgang
- Nature, Vol. 495, Issue 7439
Base-Free Methanol Dehydrogenation Using a Pincer-Supported Iron Compound and Lewis Acid Co-catalyst
journal, March 2015
- Bielinski, Elizabeth A.; Förster, Moritz; Zhang, Yuanyuan
- ACS Catalysis, Vol. 5, Issue 4
Lewis Acid-Assisted Formic Acid Dehydrogenation Using a Pincer-Supported Iron Catalyst
journal, July 2014
- Bielinski, Elizabeth A.; Lagaditis, Paraskevi O.; Zhang, Yuanyuan
- Journal of the American Chemical Society, Vol. 136, Issue 29
Synthesis and Structure of Six-Coordinate Iron Borohydride Complexes Supported by PNP Ligands
journal, December 2013
- Koehne, Ingo; Schmeier, Timothy J.; Bielinski, Elizabeth A.
- Inorganic Chemistry, Vol. 53, Issue 4
Well-Defined Iron Catalysts for the Acceptorless Reversible Dehydrogenation-Hydrogenation of Alcohols and Ketones
journal, October 2014
- Chakraborty, Sumit; Lagaditis, Paraskevi O.; Förster, Moritz
- ACS Catalysis, Vol. 4, Issue 11
The synthesis of PNP-supported low-spin nitro manganese(I) carbonyl complexes
journal, September 2016
- Tondreau, Aaron M.; Boncella, James M.
- Polyhedron, Vol. 116
Ligand Reactivity in Diarylamido/Bis(Phosphine) PNP Complexes of Mn(CO) 3 and Re(CO) 3
journal, October 2009
- Radosevich, Alexander T.; Melnick, Jonathan G.; Stoian, Sebastian A.
- Inorganic Chemistry, Vol. 48, Issue 19
High-Spin Manganese(II) Complexes of an Amido/Bis(Phosphine) PNP Ligand
journal, May 2010
- Bacciu, Deborha; Chen, Chun-Hsing; Surawatanawong, Panida
- Inorganic Chemistry, Vol. 49, Issue 11
Ruthenium Complexes with Cooperative PNP-Pincer Amine, Amido, Imine, and Enamido Ligands: Facile Ligand Backbone Functionalization Processes
journal, June 2010
- Friedrich, Anja; Drees, Markus; Käss, Martina
- Inorganic Chemistry, Vol. 49, Issue 12
Bacillus subtilis YvrK Is an Acid-Induced Oxalate Decarboxylase
journal, January 2000
- Tanner, Adam; Bornemann, Stephen
- Journal of Bacteriology, Vol. 182, Issue 18
Oxalate Decarboxylase Requires Manganese and Dioxygen for Activity: OVEREXPRESSION AND CHARACTERIZATION OF
journal, August 2001
- Tanner, Adam; Bowater, Laura; Fairhurst, Shirley A.
- Journal of Biological Chemistry, Vol. 276, Issue 47
Structure of Oxalate Decarboxylase from Bacillus subtilis at 1.75 Å Resolution † , ‡
journal, June 2002
- Anand, Ruchi; Dorrestein, Pieter C.; Kinsland, Cynthia
- Biochemistry, Vol. 41, Issue 24
A Closed Conformation of Bacillus subtilis Oxalate Decarboxylase OxdC Provides Evidence for the True Identity of the Active Site
journal, May 2004
- Just, Victoria J.; Stevenson, Clare E. M.; Bowater, Laura
- Journal of Biological Chemistry, Vol. 279, Issue 19
Homogeneous catalytic hydrogenation of free carboxylic acids in the presence of cluster ruthenium carbonyl hydrides
journal, April 1980
- Bianchi, Mario; Menchi, Gloria; Francalanci, Franco
- Journal of Organometallic Chemistry, Vol. 188, Issue 1
Iron catalyzed CO 2 hydrogenation to formate enhanced by Lewis acid co-catalysts
journal, January 2015
- Zhang, Yuanyuan; MacIntosh, Alex D.; Wong, Janice L.
- Chemical Science, Vol. 6, Issue 7
Synthesis and characterization of iron complexes based on bis-phosphinite PONOP and bis-phosphite PONOP pincer ligands
journal, December 2014
- DeRieux, Wing-Sy W.; Wong, Aaron; Schrodi, Yann
- Journal of Organometallic Chemistry, Vol. 772-773
Works referencing / citing this record:
Bio-mimetic self-assembled computationally designed catalysts of Mo and W for hydrogenation of CO 2 /dehydrogenation of HCOOH inspired by the active site of formate dehydrogenase
journal, January 2019
- Shiekh, Bilal Ahmad; Kaur, Damanjit; Kumar, Sourav
- Physical Chemistry Chemical Physics, Vol. 21, Issue 38
Manganese-Catalyzed Sustainable Synthesis of Pyrroles from Alcohols and Amino Alcohols
journal, May 2017
- Kallmeier, Fabian; Dudziec, Beata; Irrgang, Torsten
- Angewandte Chemie International Edition, Vol. 56, Issue 25
Benzylene-linked [PNP] scaffolds and their cyclometalated zirconium and hafnium complexes
journal, January 2017
- Sietzen, Malte; Batke, Sonja; Antoni, Patrick W.
- Dalton Transactions, Vol. 46, Issue 18
Catalytic upgrading of ethanol to n -butanol using an aliphatic Mn–PNP complex: theoretical insights into reaction mechanisms and product selectivity
journal, January 2019
- Rawat, Kuber Singh; Mandal, Shyama Charan; Bhauriyal, Preeti
- Catalysis Science & Technology, Vol. 9, Issue 11
Hydrogenation of Carbonyl Derivatives Catalysed by Manganese Complexes Bearing Bidentate Pyridinyl-Phosphine Ligands
journal, December 2017
- Wei, Duo; Bruneau-Voisine, Antoine; Chauvin, Téo
- Advanced Synthesis & Catalysis, Vol. 360, Issue 4
Oxidative Transformations of Biosourced Alcohols Catalyzed by Earth-Abundant Transition Metals
journal, June 2017
- Nguyen, Duc Hanh; Morin, Yohann; Zhang, Lei
- ChemCatChem, Vol. 9, Issue 14
Mangankomplexe in der (De)Hydrier-Katalyse - ein Vergleich mit Cobalt- und Eisenkatalysatoren
journal, December 2017
- Kallmeier, Fabian; Kempe, Rhett
- Angewandte Chemie, Vol. 130, Issue 1
Pentacarbonylmethylmanganese( i ) as a synthon for Mn( i ) pincer catalysts
journal, January 2019
- Kadassery, Karthika J.; Lacy, David C.
- Dalton Transactions, Vol. 48, Issue 14
Manganese-Catalyzed Direct Olefination of Methyl-Substituted Heteroarenes with Primary Alcohols
journal, June 2018
- Barman, Milan K.; Waiba, Satyadeep; Maji, Biplab
- Angewandte Chemie, Vol. 130, Issue 29
Manganese Complexes for (De)Hydrogenation Catalysis: A Comparison to Cobalt and Iron Catalysts
journal, December 2017
- Kallmeier, Fabian; Kempe, Rhett
- Angewandte Chemie International Edition, Vol. 57, Issue 1
Manganese(I)-Catalyzed Enantioselective Hydrogenation of Ketones Using a Defined Chiral PNP Pincer Ligand
journal, August 2017
- Garbe, Marcel; Junge, Kathrin; Walker, Svenja
- Angewandte Chemie International Edition, Vol. 56, Issue 37
A hemilabile manganese( i )–phenol complex and its coordination induced O–H bond weakening
journal, January 2020
- Kadassery, Karthika J.; Crawley, Matthew R.; MacMillan, Samantha N.
- Dalton Transactions
A Highly Active Manganese Catalyst for Enantioselective Ketone and Ester Hydrogenation
journal, April 2017
- Widegren, Magnus B.; Harkness, Gavin J.; Slawin, Alexandra M. Z.
- Angewandte Chemie, Vol. 129, Issue 21
Highly Efficient Base-Free Dehydrogenation of Formic Acid at Low Temperature
journal, August 2018
- Prichatz, Christoph; Trincado, Monica; Tan, Lilin
- ChemSusChem, Vol. 11, Issue 18
Carbon dioxide hydrogenation catalysed by well-defined Mn( i ) PNP pincer hydride complexes
journal, January 2017
- Bertini, Federica; Glatz, Mathias; Gorgas, Nikolaus
- Chemical Science, Vol. 8, Issue 7
Synthesis and characterization of bis- and tris-carbonyl Mn(I) and Re(I) PNP pincer complexes
journal, October 2018
- Glatz, Mathias; Pecak, Jan; Haager, Lena
- Monatshefte für Chemie - Chemical Monthly, Vol. 150, Issue 1
Hydrogen production from formic acid catalyzed by a phosphine free manganese complex: investigation and mechanistic insights
journal, January 2020
- Léval, Alexander; Agapova, Anastasiya; Steinlechner, Christoph
- Green Chemistry, Vol. 22, Issue 3
Benyzl Alcohol Dehydrogenative Coupling Catalyzed by Defined Mn and Re PNP Pincer Complexes - A Computational Mechanistic Study: Benyzl Alcohol Dehydrogenative Coupling Catalyzed by Defined Mn and Re PNP Pincer Complexes - A Computational Mechanistic Study
journal, November 2018
- Wei, Zhihong; de Aguirre, Adiran; Junge, Kathrin
- European Journal of Inorganic Chemistry, Vol. 2018, Issue 42
Manganese(I)-Catalyzed Enantioselective Hydrogenation of Ketones Using a Defined Chiral PNP Pincer Ligand
journal, August 2017
- Garbe, Marcel; Junge, Kathrin; Walker, Svenja
- Angewandte Chemie, Vol. 129, Issue 37
A Highly Active Manganese Catalyst for Enantioselective Ketone and Ester Hydrogenation
journal, April 2017
- Widegren, Magnus B.; Harkness, Gavin J.; Slawin, Alexandra M. Z.
- Angewandte Chemie International Edition, Vol. 56, Issue 21
Manganese-Catalyzed Direct Olefination of Methyl-Substituted Heteroarenes with Primary Alcohols
journal, June 2018
- Barman, Milan K.; Waiba, Satyadeep; Maji, Biplab
- Angewandte Chemie International Edition, Vol. 57, Issue 29
Synthesis and characterization of bis- and tris-carbonyl Mn(I) and Re(I) PNP pincer complexes
journal, October 2018
- Glatz, Mathias; Pecak, Jan; Haager, Lena
- Monatshefte für Chemie - Chemical Monthly, Vol. 150, Issue 1
Manganese(I)-Catalyzed Cross-Coupling of Ketones and Secondary Alcohols with Primary Alcohols
journal, June 2019
- Gawali, Suhas Shahaji; Pandia, Biplab Keshari; Pal, Souvik
- ACS Omega, Vol. 4, Issue 6
Figures / Tables found in this record:

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal