Hydrogenation properties of lithium and sodium hydride – closo -borate, [B 10 H 10 ] 2− and [B 12 H 12 ] 2− , composites
Abstract
The hydrogen absorption properties of metal closo-borate metal hydride composites, M2B10H10– 8MH and M2B12H12–10MH, M = Li or Na, are studied under high hydrogen pressures to understand the formation mechanism of metal borohydrides. The hydrogen storage properties of the composites have been investigated by in-situ synchrotron radiation powder X-ray diffraction at p(H2) = 400 bar and by ex-situ hydrogen absorption measurements at p(H2) = 526 to 998 bar. The in-situ experiments reveal the formation of crystalline intermediates before metal borohydrides (MBH4) are formed. The M2B12H12–10MH (M = Li and Na) systems show no formation of the metal borohydride at T = 400 °C and p(H2) = 537 to 970 bar. 11B MAS NMR of the M2B10H10– 8MH composites reveal that the molar ratio of LiBH4 or NaBH4 and the remaining B species is 1:0.63 and 1:0.21, respectively. Solution and solid-state 11B NMR spectra reveal new intermediates with a B:H ratio close to 1:1. Our results indicate that the M2B10H10 (M = Li, Na) salts display a higher reactivity towards hydrogen in the presence of metal hydrides compared to the corresponding [B12H12]2- compounds, which represents an important step towards understanding the factors that determine the stability and reversibility of high hydrogenmore »
- Authors:
-
- Center for Materials Crystallography, Interdisciplinary Nanoscience Center (iNANO) and Department of Chemistry, Aarhus University, 8000 Aarhus C, Denmark
- Chemistry, Combustion, and Materials Center, Sandia National Laboratories, Livermore, USA
- Department of Chemistry and Interdisciplinary Nanoscience Center (iNANO), Aarhus University, 8000 Aarhus C, Denmark
- Publication Date:
- Research Org.:
- Sandia National Lab. (SNL-NM), Albuquerque, NM (United States); Sandia National Lab. (SNL-CA), Livermore, CA (United States)
- Sponsoring Org.:
- USDOE National Nuclear Security Administration (NNSA); USDOE Office of Energy Efficiency and Renewable Energy (EERE), Sustainable Transportation Office. Hydrogen Fuel Cell Technologies Office (HFTO)
- OSTI Identifier:
- 1470579
- Alternate Identifier(s):
- OSTI ID: 1429663; OSTI ID: 1440264; OSTI ID: 1444088
- Report Number(s):
- SAND-2017-11502J; SAND-2018-6006J
Journal ID: ISSN 1463-9076; PPCPFQ
- Grant/Contract Number:
- AC04-94AL85000; NA0003525
- Resource Type:
- Published Article
- Journal Name:
- Physical Chemistry Chemical Physics. PCCP
- Additional Journal Information:
- Journal Name: Physical Chemistry Chemical Physics. PCCP Journal Volume: 20 Journal Issue: 23; Journal ID: ISSN 1463-9076
- Publisher:
- Royal Society of Chemistry (RSC)
- Country of Publication:
- United Kingdom
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY; 08 HYDROGEN
Citation Formats
Jensen, Steffen R. H., Paskevicius, Mark, Hansen, Bjarne R. S., Jakobsen, Anders S., Møller, Kasper T., White, James L., Allendorf, Mark D., Stavila, Vitalie, Skibsted, Jørgen, and Jensen, Torben R. Hydrogenation properties of lithium and sodium hydride – closo -borate, [B 10 H 10 ] 2− and [B 12 H 12 ] 2− , composites. United Kingdom: N. p., 2018.
Web. doi:10.1039/C7CP07776A.
Jensen, Steffen R. H., Paskevicius, Mark, Hansen, Bjarne R. S., Jakobsen, Anders S., Møller, Kasper T., White, James L., Allendorf, Mark D., Stavila, Vitalie, Skibsted, Jørgen, & Jensen, Torben R. Hydrogenation properties of lithium and sodium hydride – closo -borate, [B 10 H 10 ] 2− and [B 12 H 12 ] 2− , composites. United Kingdom. https://doi.org/10.1039/C7CP07776A
Jensen, Steffen R. H., Paskevicius, Mark, Hansen, Bjarne R. S., Jakobsen, Anders S., Møller, Kasper T., White, James L., Allendorf, Mark D., Stavila, Vitalie, Skibsted, Jørgen, and Jensen, Torben R. Mon .
"Hydrogenation properties of lithium and sodium hydride – closo -borate, [B 10 H 10 ] 2− and [B 12 H 12 ] 2− , composites". United Kingdom. https://doi.org/10.1039/C7CP07776A.
@article{osti_1470579,
title = {Hydrogenation properties of lithium and sodium hydride – closo -borate, [B 10 H 10 ] 2− and [B 12 H 12 ] 2− , composites},
author = {Jensen, Steffen R. H. and Paskevicius, Mark and Hansen, Bjarne R. S. and Jakobsen, Anders S. and Møller, Kasper T. and White, James L. and Allendorf, Mark D. and Stavila, Vitalie and Skibsted, Jørgen and Jensen, Torben R.},
abstractNote = {The hydrogen absorption properties of metal closo-borate metal hydride composites, M2B10H10– 8MH and M2B12H12–10MH, M = Li or Na, are studied under high hydrogen pressures to understand the formation mechanism of metal borohydrides. The hydrogen storage properties of the composites have been investigated by in-situ synchrotron radiation powder X-ray diffraction at p(H2) = 400 bar and by ex-situ hydrogen absorption measurements at p(H2) = 526 to 998 bar. The in-situ experiments reveal the formation of crystalline intermediates before metal borohydrides (MBH4) are formed. The M2B12H12–10MH (M = Li and Na) systems show no formation of the metal borohydride at T = 400 °C and p(H2) = 537 to 970 bar. 11B MAS NMR of the M2B10H10– 8MH composites reveal that the molar ratio of LiBH4 or NaBH4 and the remaining B species is 1:0.63 and 1:0.21, respectively. Solution and solid-state 11B NMR spectra reveal new intermediates with a B:H ratio close to 1:1. Our results indicate that the M2B10H10 (M = Li, Na) salts display a higher reactivity towards hydrogen in the presence of metal hydrides compared to the corresponding [B12H12]2- compounds, which represents an important step towards understanding the factors that determine the stability and reversibility of high hydrogen capacity metal borohydrides for hydrogen storage.},
doi = {10.1039/C7CP07776A},
journal = {Physical Chemistry Chemical Physics. PCCP},
number = 23,
volume = 20,
place = {United Kingdom},
year = {Mon Jan 01 00:00:00 EST 2018},
month = {Mon Jan 01 00:00:00 EST 2018}
}
https://doi.org/10.1039/C7CP07776A
Web of Science
Figures / Tables:
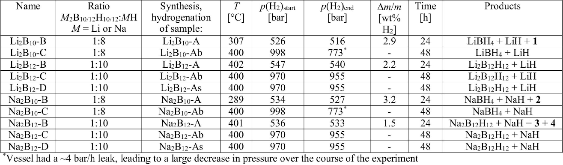 Table 1: Overview of the hydrogenated samples. The total pressure allows calculation of hydrogen H2 uptake (Δm/m). Unidentified compounds are denoted, 1, 2, 3, or 4.
Table 1: Overview of the hydrogenated samples. The total pressure allows calculation of hydrogen H2 uptake (Δm/m). Unidentified compounds are denoted, 1, 2, 3, or 4.
Works referenced in this record:
Probing the structure, stability and hydrogen storage properties of calcium dodecahydro-closo-dodecaborate
journal, May 2010
- Stavila, Vitalie; Her, Jae-Hyuk; Zhou, Wei
- Journal of Solid State Chemistry, Vol. 183, Issue 5
Hydrogen Storage Materials for Mobile and Stationary Applications: Current State of the Art
journal, June 2015
- Lai, Qiwen; Paskevicius, Mark; Sheppard, Drew A.
- ChemSusChem, Vol. 8, Issue 17
Recent Progress in Metal Borohydrides for Hydrogen Storage
journal, January 2011
- Li, Hai-Wen; Yan, Yigang; Orimo, Shin-ichi
- Energies, Vol. 4, Issue 1
In situ X-ray diffraction environments for high-pressure reactions
journal, July 2015
- Hansen, Bjarne R. S.; Møller, Kasper T.; Paskevicius, Mark
- Journal of Applied Crystallography, Vol. 48, Issue 4
Role of Li 2 B 12 H 12 for the Formation and Decomposition of LiBH 4
journal, May 2010
- Friedrichs, O.; Remhof, A.; Hwang, S. -J.
- Chemistry of Materials, Vol. 22, Issue 10
Exceptional Superionic Conductivity in Disordered Sodium Decahydro- closo -decaborate
journal, October 2014
- Udovic, Terrence J.; Matsuo, Motoaki; Tang, Wan Si
- Advanced Materials, Vol. 26, Issue 45
Structure and properties of complex hydride perovskite materials
journal, December 2014
- Schouwink, Pascal; Ley, Morten B.; Tissot, Antoine
- Nature Communications, Vol. 5, Issue 1
Tetrahydroborates: Development and Potential as Hydrogen Storage Medium
journal, October 2017
- Puszkiel, Julián; Garroni, Sebastiano; Milanese, Chiara
- Inorganics, Vol. 5, Issue 4
Chemistry of Boranes. VIII. Salts and Acids of B 10 H 10 -2 and B 12 12 -2
journal, March 1964
- Muetterties, E. L.; Balthis, J. H.; Chia, Y. T.
- Inorganic Chemistry, Vol. 3, Issue 3
The role of MgB 12 H 12 in the hydrogen desorption process of Mg(BH 4 ) 2
journal, January 2015
- Yan, Yigang; Remhof, Arndt; Rentsch, Daniel
- Chemical Communications, Vol. 51, Issue 4
Trimetallic Borohydride Li 3 MZn 5 (BH 4 ) 15 (M = Mg, Mn) Containing Two Weakly Interconnected Frameworks
journal, August 2013
- Černý, Radovan; Schouwink, Pascal; Sadikin, Yolanda
- Inorganic Chemistry, Vol. 52, Issue 17
Theoretical Prediction of Metastable Intermediates in the Decomposition of Mg(BH 4 ) 2
journal, May 2012
- Zhang, Yongsheng; Majzoub, Eric; Ozoliņš, Vidvuds
- The Journal of Physical Chemistry C, Vol. 116, Issue 19
A High-Resolution Solid-State 23 Na NMR Study of Sodium Complexes with Solvents, Small Ligand Molecules, and Ionophores. 23 Na Chemical Shifts as Means for Identification and Characterization of Ion–Ion, Ion–Solvent, and Ion–Ligand Interactions
journal, June 1986
- Tabeta, Ryoko; Aida, Misako; Saitô, Hazime
- Bulletin of the Chemical Society of Japan, Vol. 59, Issue 6
First-principles calculated decomposition pathways for LiBH4 nanoclusters
journal, May 2016
- Huang, Zhi-Quan; Chen, Wei-Chih; Chuang, Feng-Chuan
- Scientific Reports, Vol. 6, Issue 1
Al3Li4(BH4)13: A Complex Double-Cation Borohydride with a New Structure
journal, June 2010
- Lindemann, Inge; Domènech Ferrer, Roger; Dunsch, Lothar
- Chemistry - A European Journal, Vol. 16, Issue 29
Understanding and Mitigating the Effects of Stable Dodecahydro- closo -dodecaborate Intermediates on Hydrogen-Storage Reactions
journal, November 2016
- White, James L.; Newhouse, Rebecca J.; Zhang, Jin Z.
- The Journal of Physical Chemistry C, Vol. 120, Issue 45
The versatile chemistry of the [B20H18]2– ions: novel reactions and structural motifs
journal, February 2002
- Hawthorne, M. Frederick; Shelly, Kenneth; Li, Fangbiao
- Chemical Communications, Issue 6
Thermal Decomposition of Anhydrous Alkali Metal Dodecaborates M2B12H12 (M = Li, Na, K)
journal, November 2015
- He, Liqing; Li, Hai-Wen; Akiba, Etsuo
- Energies, Vol. 8, Issue 11
Stability and Decomposition of NaBH 4
journal, March 2010
- Martelli, Pascal; Caputo, Riccarda; Remhof, Arndt
- The Journal of Physical Chemistry C, Vol. 114, Issue 15
Boron–nitrogen based hydrides and reactive composites for hydrogen storage
journal, April 2014
- Jepsen, Lars H.; Ley, Morten B.; Lee, Young-Su
- Materials Today, Vol. 17, Issue 3
Complex hydrides for hydrogen storage – new perspectives
journal, April 2014
- Ley, Morten B.; Jepsen, Lars H.; Lee, Young-Su
- Materials Today, Vol. 17, Issue 3
Novel solvates M(BH 4 ) 3 S(CH 3 ) 2 and properties of halide-free M(BH 4 ) 3 (M = Y or Gd)
journal, January 2014
- Ley, Morten B.; Paskevicius, Mark; Schouwink, Pascal
- Dalton Trans., Vol. 43, Issue 35
Metal hydride materials for solid hydrogen storage: A review☆
journal, June 2007
- Sakintuna, B.; Lamaridarkrim, F.; Hirscher, M.
- International Journal of Hydrogen Energy, Vol. 32, Issue 9
FT-IR spectra of inorganic borohydrides
journal, July 2014
- D’Anna, Vincenza; Spyratou, Alexandra; Sharma, Manish
- Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, Vol. 128
Intermediate phases observed during decomposition of LiBH4
journal, October 2007
- Mosegaard, Lene; Møller, Bitten; Jørgensen, Jens-Erik
- Journal of Alloys and Compounds, Vol. 446-447
Using first principles calculations to identify new destabilized metal hydride reactions for reversible hydrogen storage
journal, January 2007
- Alapati, Sudhakar V.; Karl Johnson, J.; Sholl, David S.
- Physical Chemistry Chemical Physics, Vol. 9, Issue 12
Crystal structure and in situ decomposition of Eu(BH 4 ) 2 and Sm(BH 4 ) 2
journal, January 2015
- Humphries, Terry D.; Ley, Morten B.; Frommen, Christoph
- Journal of Materials Chemistry A, Vol. 3, Issue 2
Boron-based hydrides for chemical hydrogen storage: Boron-based hydrolytic and thermolytic hydrides
journal, March 2013
- Moussa, Georges; Moury, Romain; Demirci, Umit B.
- International Journal of Energy Research, Vol. 37, Issue 8
Eutectic melting in metal borohydrides
journal, January 2013
- Paskevicius, Mark; Ley, Morten B.; Sheppard, Drew A.
- Physical Chemistry Chemical Physics, Vol. 15, Issue 45
Thermal Stability of Li 2 B 12 H 12 and its Role in the Decomposition of LiBH 4
journal, April 2013
- Pitt, Mark P.; Paskevicius, Mark; Brown, David H.
- Journal of the American Chemical Society, Vol. 135, Issue 18
Reversible dehydrogenation of magnesium borohydride to magnesium triborane in the solid state under moderate conditions
journal, January 2011
- Chong, Marina; Karkamkar, Abhi; Autrey, Tom
- Chem. Commun., Vol. 47, Issue 4
The crystal chemistry of inorganic metal borohydrides and their relation to metal oxides
journal, December 2015
- Černý, Radovan; Schouwink, Pascal
- Acta Crystallographica Section B Structural Science, Crystal Engineering and Materials, Vol. 71, Issue 6
Complex high-temperature phase transitions in Li2B12H12 and Na2B12H12
journal, April 2014
- Verdal, Nina; Her, Jae-Hyuk; Stavila, Vitalie
- Journal of Solid State Chemistry, Vol. 212
A review of catalyst-enhanced magnesium hydride as a hydrogen storage material
journal, September 2015
- Webb, C. J.
- Journal of Physics and Chemistry of Solids, Vol. 84
In situ solid state 11B MAS-NMR studies of the thermal decomposition of ammonia borane: mechanistic studies of the hydrogen release pathways from a solid state hydrogen storage material
journal, January 2007
- Stowe, Ashley C.; Shaw, Wendy J.; Linehan, John C.
- Physical Chemistry Chemical Physics, Vol. 9, Issue 15
Large-scale screening of metal hydrides for hydrogen storage from first-principles calculations based on equilibrium reaction thermodynamics
journal, January 2011
- Kim, Ki Chul; Kulkarni, Anant D.; Johnson, J. Karl
- Physical Chemistry Chemical Physics, Vol. 13, Issue 15
First-Principles Prediction of Thermodynamically Reversible Hydrogen Storage Reactions in the Li-Mg-Ca-B-H System
journal, January 2009
- Ozolins, V.; Majzoub, E. H.; Wolverton, C.
- Journal of the American Chemical Society, Vol. 131, Issue 1
Metallo Borohydrides. III. Lithium Borohydride
journal, December 1940
- Schlesinger, H. I.; Brown, Herbert C.
- Journal of the American Chemical Society, Vol. 62, Issue 12
Bimetallic Borohydrides in the System M (BH 4 ) 2 –KBH 4 ( M = Mg, Mn): On the Structural Diversity
journal, May 2012
- Schouwink, Pascal; D’Anna, Vincenza; Ley, Morten Brix
- The Journal of Physical Chemistry C, Vol. 116, Issue 20
Phase Diagram for the NaBH 4 –KBH 4 System and the Stability of a Na 1– x K x BH 4 Solid Solution
journal, December 2015
- Jensen, Steffen R. H.; Jepsen, Lars H.; Skibsted, Jørgen
- The Journal of Physical Chemistry C, Vol. 119, Issue 50
Metal borohydrides and derivatives – synthesis, structure and properties
journal, January 2017
- Paskevicius, Mark; Jepsen, Lars H.; Schouwink, Pascal
- Chemical Society Reviews, Vol. 46, Issue 5
Amides and borohydrides for high-capacity solid-state hydrogen storage—materials design and kinetic improvements
journal, June 2013
- Wang, Jianhui; Li, Hai-Wen; Chen, Ping
- MRS Bulletin, Vol. 38, Issue 6
Hydrogen - A sustainable energy carrier
journal, February 2017
- Møller, Kasper T.; Jensen, Torben R.; Akiba, Etsuo
- Progress in Natural Science: Materials International, Vol. 27, Issue 1
Reversible Storage of Hydrogen in Destabilized LiBH 4
journal, March 2005
- Vajo, John J.; Skeith, Sky L.; Mertens, Florian
- The Journal of Physical Chemistry B, Vol. 109, Issue 9
Theoretical study of <mml:math altimg="si1.gif" overflow="scroll" xmlns:xocs="http://www.elsevier.com/xml/xocs/dtd" xmlns:xs="http://www.w3.org/2001/XMLSchema" xmlns:xsi="http://www.w3.org/2001/XMLSchema-instance" xmlns="http://www.elsevier.com/xml/ja/dtd" xmlns:ja="http://www.elsevier.com/xml/ja/dtd" xmlns:mml="http://www.w3.org/1998/Math/MathML" xmlns:tb="http://www.elsevier.com/xml/common/table/dtd" xmlns:sb="http://www.elsevier.com/xml/common/struct-bib/dtd" xmlns:ce="http://www.elsevier.com/xml/common/dtd" xmlns:xlink="http://www.w3.org/1999/xlink" xmlns:cals="http://www.elsevier.com/xml/common/cals/dtd" xmlns:sa="http://www.elsevier.com/xml/common/struct-aff/dtd"><mml:mrow><mml:msub><mml:mi>B</mml:mi><mml:mrow><mml:mn>12</mml:mn></mml:mrow></mml:msub><mml:msub><mml:mi>H</mml:mi><mml:mi>n</mml:mi></mml:msub><mml:msubsup><mml:mi>F</mml:mi><mml:mrow><mml:mrow><mml:mo>(</mml:mo><mml:mrow><mml:mn>12</mml:mn><mml:mo>−</mml:mo><mml:mi>n</mml:mi></mml:mrow><mml:mo>)</mml:mo></mml:mrow></mml:mrow><mml:mrow><mml:mn>2</mml:mn><mml:mo>−</mml:mo></mml:mrow></mml:msubsup></mml:mrow></mml:math> species
journal, October 2015
- Sharma, Manish; Sethio, Daniel; D'Anna, Vincenza
- International Journal of Hydrogen Energy, Vol. 40, Issue 37
The effect of Al on the hydrogen sorption mechanism of LiBH4
journal, January 2009
- Friedrichs, O.; Kim, J. W.; Remhof, A.
- Physical Chemistry Chemical Physics, Vol. 11, Issue 10
Hydrogen reversibility of LiBH 4 –MgH 2 –Al composites
journal, January 2014
- Hansen, Bjarne R. S.; Ravnsbæk, Dorthe B.; Skibsted, Jørgen
- Phys. Chem. Chem. Phys., Vol. 16, Issue 19
Chemistry of Boranes. III. 1 The Infrared and Raman Spectra of B 12 H 12 - and Related Anions
journal, July 1962
- Muetterties, E. L.; Merrifield, R. E.; Miller, H. C.
- Journal of the American Chemical Society, Vol. 84, Issue 13
Metal boranes: Progress and applications
journal, September 2016
- Hansen, Bjarne R. S.; Paskevicius, Mark; Li, Hai-Wen
- Coordination Chemistry Reviews, Vol. 323
Direct hydrogenation of magnesium boride to magnesium borohydride: demonstration of >11 weight percent reversible hydrogenstorage
journal, January 2010
- Severa, Godwin; Rönnebro, Ewa; Jensen, Craig M.
- Chem. Commun., Vol. 46, Issue 3
Ammine-Stabilized Transition-Metal Borohydrides of Iron, Cobalt, and Chromium: Synthesis and Characterization
journal, October 2015
- Roedern, Elsa; Jensen, Torben R.
- Inorganic Chemistry, Vol. 54, Issue 21
Thermodynamic Changes in Mechanochemically Synthesized Magnesium Hydride Nanoparticles
journal, April 2010
- Paskevicius, Mark; Sheppard, Drew A.; Buckley, Craig E.
- Journal of the American Chemical Society, Vol. 132, Issue 14

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal