Manganese–Cobalt Oxido Cubanes Relevant to Manganese-Doped Water Oxidation Catalysts
Abstract
Incorporation of Mn into an established water oxidation catalyst based on a Co(III)4O4 cubane was achieved by a simple and efficient assembly of permanganate, cobalt(II) acetate, and pyridine to form the cubane oxo cluster MnCo3O4(OAc)5py3 (OAc = acetate, py = pyridine) (1-OAc) in good yield. This allows characterization of electronic and chemical properties for a manganese center in a cobalt oxide environment, and provides a molecular model for Mn-doped cobalt oxides. The electronic properties of the cubane are readily tuned by exchange of the OAc- ligand for Cl- (1-Cl), NO3- (1-NO3), and pyridine ([1-py]+). EPR spectroscopy, SQUID magnetometry, and DFT calculations thoroughly characterized the valence assignment of the cubane as [MnIVCoIII3]. These cubanes are redox-active, and calculations reveal that the Co ions behave as the reservoir for electrons, but their redox potentials are tuned by the choice of ligand at Mn. This MnCo3O4 cubane system represents a new class of easily prepared, versatile, and redox-active oxido clusters that should contribute to an understanding of mixed-metal, Mn-containing oxides.
- Authors:
-
- Univ. of California, Berkeley, CA (United States). Dept. of Chemistry
- Univ. of California, Davis, CA (United States). Dept. of Chemistry
- Publication Date:
- Research Org.:
- Univ. of California, Berkeley, CA (United States)
- Sponsoring Org.:
- USDOE Office of Science (SC), Basic Energy Sciences (BES). Chemical Sciences, Geosciences, and Biosciences Division; National Science Foundation (NSF); National Institutes of Health (NIH)
- OSTI Identifier:
- 1469819
- Grant/Contract Number:
- AC02-05CH11231; FG02-11ER16282; S10-RR027172
- Resource Type:
- Accepted Manuscript
- Journal Name:
- Journal of the American Chemical Society
- Additional Journal Information:
- Journal Volume: 139; Journal Issue: 15; Journal ID: ISSN 0002-7863
- Publisher:
- American Chemical Society (ACS)
- Country of Publication:
- United States
- Language:
- English
- Subject:
- 37 INORGANIC, ORGANIC, PHYSICAL, AND ANALYTICAL CHEMISTRY
Citation Formats
Nguyen, Andy I., Suess, Daniel L. M., Darago, Lucy E., Oyala, Paul H., Levine, Daniel S., Ziegler, Micah S., Britt, R. David, and Tilley, T. Don. Manganese–Cobalt Oxido Cubanes Relevant to Manganese-Doped Water Oxidation Catalysts. United States: N. p., 2017.
Web. doi:10.1021/jacs.7b01792.
Nguyen, Andy I., Suess, Daniel L. M., Darago, Lucy E., Oyala, Paul H., Levine, Daniel S., Ziegler, Micah S., Britt, R. David, & Tilley, T. Don. Manganese–Cobalt Oxido Cubanes Relevant to Manganese-Doped Water Oxidation Catalysts. United States. https://doi.org/10.1021/jacs.7b01792
Nguyen, Andy I., Suess, Daniel L. M., Darago, Lucy E., Oyala, Paul H., Levine, Daniel S., Ziegler, Micah S., Britt, R. David, and Tilley, T. Don. Tue .
"Manganese–Cobalt Oxido Cubanes Relevant to Manganese-Doped Water Oxidation Catalysts". United States. https://doi.org/10.1021/jacs.7b01792. https://www.osti.gov/servlets/purl/1469819.
@article{osti_1469819,
title = {Manganese–Cobalt Oxido Cubanes Relevant to Manganese-Doped Water Oxidation Catalysts},
author = {Nguyen, Andy I. and Suess, Daniel L. M. and Darago, Lucy E. and Oyala, Paul H. and Levine, Daniel S. and Ziegler, Micah S. and Britt, R. David and Tilley, T. Don},
abstractNote = {Incorporation of Mn into an established water oxidation catalyst based on a Co(III)4O4 cubane was achieved by a simple and efficient assembly of permanganate, cobalt(II) acetate, and pyridine to form the cubane oxo cluster MnCo3O4(OAc)5py3 (OAc = acetate, py = pyridine) (1-OAc) in good yield. This allows characterization of electronic and chemical properties for a manganese center in a cobalt oxide environment, and provides a molecular model for Mn-doped cobalt oxides. The electronic properties of the cubane are readily tuned by exchange of the OAc- ligand for Cl- (1-Cl), NO3- (1-NO3), and pyridine ([1-py]+). EPR spectroscopy, SQUID magnetometry, and DFT calculations thoroughly characterized the valence assignment of the cubane as [MnIVCoIII3]. These cubanes are redox-active, and calculations reveal that the Co ions behave as the reservoir for electrons, but their redox potentials are tuned by the choice of ligand at Mn. This MnCo3O4 cubane system represents a new class of easily prepared, versatile, and redox-active oxido clusters that should contribute to an understanding of mixed-metal, Mn-containing oxides.},
doi = {10.1021/jacs.7b01792},
journal = {Journal of the American Chemical Society},
number = 15,
volume = 139,
place = {United States},
year = {Tue Mar 28 00:00:00 EDT 2017},
month = {Tue Mar 28 00:00:00 EDT 2017}
}
Web of Science
Figures / Tables:
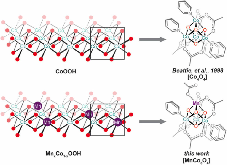 Figure 1: Comparison of models for cobalt oxyhydroxide and manganese-doped-cobalt oxyhydroxide.
Figure 1: Comparison of models for cobalt oxyhydroxide and manganese-doped-cobalt oxyhydroxide.
Works referenced in this record:
Powering the planet: Chemical challenges in solar energy utilization
journal, October 2006
- Lewis, N. S.; Nocera, D. G.
- Proceedings of the National Academy of Sciences, Vol. 103, Issue 43, p. 15729-15735
Cobalt–Iron (Oxy)hydroxide Oxygen Evolution Electrocatalysts: The Role of Structure and Composition on Activity, Stability, and Mechanism
journal, March 2015
- Burke, Michaela S.; Kast, Matthew G.; Trotochaud, Lena
- Journal of the American Chemical Society, Vol. 137, Issue 10
Nickel–Iron Oxyhydroxide Oxygen-Evolution Electrocatalysts: The Role of Intentional and Incidental Iron Incorporation
journal, April 2014
- Trotochaud, Lena; Young, Samantha L.; Ranney, James K.
- Journal of the American Chemical Society, Vol. 136, Issue 18
Ultrathin Cobalt–Manganese Layered Double Hydroxide Is an Efficient Oxygen Evolution Catalyst
journal, November 2014
- Song, Fang; Hu, Xile
- Journal of the American Chemical Society, Vol. 136, Issue 47
Efficient Water Oxidation Using CoMnP Nanoparticles
journal, March 2016
- Li, Da; Baydoun, Habib; Verani, Cláudio N.
- Journal of the American Chemical Society, Vol. 138, Issue 12
Crystal structure of oxygen-evolving photosystem II at a resolution of 1.9 Å
journal, April 2011
- Umena, Yasufumi; Kawakami, Keisuke; Shen, Jian-Ren
- Nature, Vol. 473, Issue 7345
Three-dimensional structure of the plant photosystem II reaction centre at 8 Å resolution
journal, November 1998
- Rhee, Kyong-Hi; Morris, Edward P.; Barber, James
- Nature, Vol. 396, Issue 6708
A Synthetic Model of the Mn3Ca Subsite of the Oxygen-Evolving Complex in Photosystem II
journal, August 2011
- Kanady, J. S.; Tsui, E. Y.; Day, M. W.
- Science, Vol. 333, Issue 6043
Reduction potentials of heterometallic manganese-oxido cubane complexes modulated by redox-inactive metals
journal, June 2013
- Tsui, E. Y.; Agapie, T.
- Proceedings of the National Academy of Sciences, Vol. 110, Issue 25
Mechanistic Investigations of Water Oxidation by a Molecular Cobalt Oxide Analogue: Evidence for a Highly Oxidized Intermediate and Exclusive Terminal Oxo Participation
journal, September 2015
- Nguyen, Andy I.; Ziegler, Micah S.; Oña-Burgos, Pascual
- Journal of the American Chemical Society, Vol. 137, Issue 40
Electronic Structure Description of a [Co(III) 3 Co(IV)O 4 ] Cluster: A Model for the Paramagnetic Intermediate in Cobalt-Catalyzed Water Oxidation
journal, October 2011
- McAlpin, J. Gregory; Stich, Troy A.; Ohlin, C. André
- Journal of the American Chemical Society, Vol. 133, Issue 39
Synthetic model of the asymmetric [Mn3CaO4] cubane core of the oxygen-evolving complex of photosystem II
journal, January 2012
- Mukherjee, S.; Stull, J. A.; Yano, J.
- Proceedings of the National Academy of Sciences, Vol. 109, Issue 7
A synthetic Mn4Ca-cluster mimicking the oxygen-evolving center of photosynthesis
journal, May 2015
- Zhang, C.; Chen, C.; Dong, H.
- Science, Vol. 348, Issue 6235
Water Oxidation by the [Co 4 O 4 (OAc) 4 (py) 4 ] + Cubium is Initiated by OH – Addition
journal, December 2015
- Smith, Paul F.; Hunt, Liam; Laursen, Anders B.
- Journal of the American Chemical Society, Vol. 137, Issue 49
Preparation, structure, and magnetic properties of a dodecanuclear mixed-valence manganese carboxylate
journal, September 1980
- Lis, T.
- Acta Crystallographica Section B Structural Crystallography and Crystal Chemistry, Vol. 36, Issue 9
The chemistry of cobalt acetate—IV. The isolation and crystal structure of the symmetric cubane, tetrakis[(μ-acetato)(μ3-oxo) (pyridine)cobalt(III)] · chloroform solvate, [Co4(μ3-O)4(μ-CH3CO2)4(C5H5N)in4] · 5CHCl3 and of the dicationic partial cubane, trimeric, [(μ-acetato)(acetato)tris(μ-hdyroxy(μ3-oxo) hexakispyridinetricobalt(III)]hexafluorophosphate · water solvate, [Co3(μ3-O)(μ-OH)3(μ-CH3CO2(CH3CO2(C5H5N)6[PF6]2 · 2H2O
journal, January 1998
- Beattie, James K.; Hambley, Trevor W.; Klepetko, John A.
- Polyhedron, Vol. 17, Issue 8
Synthesis, Structure, Spectral and Electrochemical Properties, and Catalytic Use of Cobalt(III)−Oxo Cubane Clusters
journal, October 2007
- Chakrabarty, Rajesh; Bora, Sanchay J.; Das, Birinchi K.
- Inorganic Chemistry, Vol. 46, Issue 22
Oxygen Atom Transfer and Oxidative Water Incorporation in Cuboidal Mn 3 MO n Complexes Based on Synthetic, Isotopic Labeling, and Computational Studies
journal, January 2013
- Kanady, Jacob S.; Mendoza-Cortes, Jose L.; Tsui, Emily Y.
- Journal of the American Chemical Society, Vol. 135, Issue 3
Role of oxido incorporation and ligand lability in expanding redox accessibility of structurally related Mn4 clusters
journal, January 2013
- Kanady, Jacob S.; Tran, Rosalie; Stull, Jamie A.
- Chemical Science, Vol. 4, Issue 10
Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion
journal, September 2015
- Shinagawa, Tatsuya; Garcia-Esparza, Angel T.; Takanabe, Kazuhiro
- Scientific Reports, Vol. 5, Issue 1
Trinucleating Copper: Synthesis and Magnetostructural Characterization of Complexes Supported by a Hexapyridyl 1,3,5-Triarylbenzene Ligand
journal, January 2011
- Tsui, Emily Y.; Day, Michael W.; Agapie, Theodor
- Angewandte Chemie International Edition, Vol. 50, Issue 7
Water Oxidation Catalysis by Co(II) Impurities in Co(III) 4 O 4 Cubanes
journal, December 2014
- Ullman, Andrew M.; Liu, Yi; Huynh, Michael
- Journal of the American Chemical Society, Vol. 136, Issue 50
Equilibrium acidities in dimethyl sulfoxide solution
journal, December 1988
- Bordwell, Frederick G.
- Accounts of Chemical Research, Vol. 21, Issue 12
Origin of the Zero-Field Splitting in Mononuclear Octahedral Mn IV Complexes: A Combined Experimental and Theoretical Investigation
journal, January 2016
- Zlatar, Matija; Gruden, Maja; Vassilyeva, Olga Yu
- Inorganic Chemistry, Vol. 55, Issue 3
55Mn ESE-ENDOR of a Mixed Valence Mn(III)Mn(IV) Complex: Comparison with the Mn Cluster of the Photosynthetic Oxygen-Evolving Complex
journal, November 1995
- Randall, David W.; Sturgeon, Bradley E.; Ball, James A.
- Journal of the American Chemical Society, Vol. 117, Issue 47
Determination and prediction of the magnetic anisotropy of Mn ions
journal, January 2016
- Duboc, Carole
- Chemical Society Reviews, Vol. 45, Issue 21
X-Band Electron Paramagnetic Resonance Comparison of Mononuclear Mn IV -oxo and Mn IV -hydroxo Complexes and Quantum Chemical Investigation of Mn IV Zero-Field Splitting
journal, March 2016
- Leto, Domenick F.; Massie, Allyssa A.; Colmer, Hannah E.
- Inorganic Chemistry, Vol. 55, Issue 7
Pulsed ELDOR detected NMR
journal, July 1994
- Schosseler, P.; Wacker, Th.; Schweiger, A.
- Chemical Physics Letters, Vol. 224, Issue 3-4
W-band ELDOR-detected NMR (EDNMR) spectroscopy as a versatile technique for the characterisation of transition metal–ligand interactions
journal, May 2013
- Cox, Nicholas; Lubitz, Wolfgang; Savitsky, Anton
- Molecular Physics, Vol. 111, Issue 18-19
LOBA: a localized orbital bonding analysis to calculate oxidation states, with application to a model water oxidation catalyst
journal, January 2009
- Thom, Alex J. W.; Sundstrom, Eric J.; Head-Gordon, Martin
- Physical Chemistry Chemical Physics, Vol. 11, Issue 47
X-ray Spectroscopic Characterization of Co(IV) and Metal–Metal Interactions in Co 4 O 4 : Electronic Structure Contributions to the Formation of High-Valent States Relevant to the Oxygen Evolution Reaction
journal, August 2016
- Hadt, Ryan G.; Hayes, Dugan; Brodsky, Casey N.
- Journal of the American Chemical Society, Vol. 138, Issue 34
Nucleophilic Attack of Hydroxide on a Mn V Oxo Complex: A Model of the O−O Bond Formation in the Oxygen Evolving Complex of Photosystem II
journal, July 2009
- Gao, Yan; Åkermark, Torbjörn; Liu, Jianhui
- Journal of the American Chemical Society, Vol. 131, Issue 25
Electronic Design Criteria for O−O Bond Formation via Metal−Oxo Complexes
journal, March 2008
- Betley, Theodore A.; Wu, Qin; Van Voorhis, Troy
- Inorganic Chemistry, Vol. 47, Issue 6
Nitric Oxide Activation by Distal Redox Modulation in Tetranuclear Iron Nitrosyl Complexes
journal, November 2015
- de Ruiter, Graham; Thompson, Niklas B.; Lionetti, Davide
- Journal of the American Chemical Society, Vol. 137, Issue 44
Works referencing / citing this record:
Half-sandwich Ir (III) and Rh (III) complexes as catalysts for water oxidation with double-site: Half-sandwich catalysts for water oxidation
journal, June 2019
- Li, Peng; Liu, Jin-Bao; Han, Sheng
- Applied Organometallic Chemistry
Co-existence of ferro- and antiferromagnetic interactions in a hexanuclear mixed-valence CoIII2MnII2MnIV2 cluster sustained by a multidentate Schiff base ligand
journal, January 2019
- Stetsiuk, Oleh; Synytsia, Valentyn; Petrusenko, Svitlana R.
- Dalton Transactions, Vol. 48, Issue 31
Heterometallic mixed-valence complex with a {Co II Co III Cu 2 O 4 } core as a new type of cobalt-based oxide cubane
journal, January 2018
- Stetsiuk, Oleh; El-Ghayoury, Abdelkrim; Kokozay, Vladimir N.
- Journal of Coordination Chemistry, Vol. 71, Issue 1
Smoothing the single-crystal to single-crystal conversions of a two-dimensional metal–organic framework via the hetero-metal doping of the linear trimetallic secondary building unit
journal, January 2018
- Chao, Meng-Yao; Chen, Jing; Young, David J.
- Dalton Transactions, Vol. 47, Issue 38

 Search WorldCat to find libraries that may hold this journal
Search WorldCat to find libraries that may hold this journal